Products
-

Giardia Lamblia Antigen Rapid Test
REF 501100 Specification 1、20 Test/box Detection principle Antigen Specimens Fecal matter(cat/dog) Intended Use This product is used for rapid screening of pet dog and cat faecal samples for Giardia lamblia antigen, and can be used as an aid in the diagnosis of Giardia lamblia. -

Pet Detection
Pet detection product is online.
Please contact us if you need it.
Nina Wang:sales@limingbio.com
Vicky Chen:vickychen@limingbio.com
-

Fetal Fibronectin Rapid Test
REF 500160 Specification 20 Tests/Box Detection principle Immunochromatographic assay Specimens Cervicovaginal secretions Intended Use StrongStep® Fetal Fibronectin Rapid Test is a visually interpreted immunochromatographic test intended to be used for the qualitative detection of fetal fibronectin in cervicovaginal secretions. -

Dermatophytosis rapid diagnostic kit
REF 500280 Specification 20 Test/box Detection principle Immunochromatographic assay Specimens Swab/ Nails/ Scurf/ Hair Intended Use The StrongStep®Dermatophytosis Diagnostic kit is a rapid visual immunoassay for the qualitative presumptive detection of α-1, 6 mannose in fungi belonging to dermatophytes. This kit is intended to be used as an aid in the diagnosis of Dermatophytosis. -

Candida albicans/Trichomonas Vaginalis/Gardnerella Vaginalis Antigen Combo Rapid Test
REF 500340 Specification 20 Test/box Detection principle Immunochromatographic assay Specimens Vulvovaginal candidiasis/Trichomonal vaginitis/Bacterial vaginosis Intended Use For the qualitative detection of Trichomonas and/or Candida and/or Gardnerella Vaginalis antigens from vaginal swabs or from the saline solution prepared when making wet mount from vaginal swabs. This kit is intended to be used as an aid in the diagnosis of Candida albicans and/or Trichomonas Vaginalis andlorGardnerella Vaginalis infection. -

Chlamydia Trachomatis Antigen Rapid Test
REF 500010 Specification 20 Tests/Box Detection principle Immunochromatographic assay Specimens Cervical/urethra swab
Intended Use This is a rapid lateral-flow immunoassay for the qualitative presumptive detection of Chlamydia trachomatis antigen in male urethral and female cervical swab. -

Gardnerella Vaginalis Antigen Rapid Test
REF 500330 Specification 20 Test/box Detection principle Immunochromatographic assay Specimens Vaginal discharge Intended Use For the qualitative detection of Gardnerella Vaginalis from vaginal swabs or from the saline solution prepared when making wet mount from vaginal swabs. This kit is intended to be used as an aid in the diagnosis of Gardnerella Vaginalis infection. -
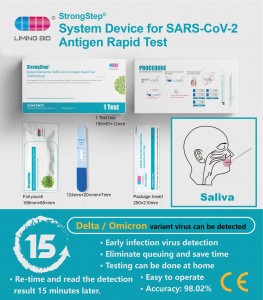
StrongStep System Device for SARS-CoV-2 Antigen Rapid Test
REF 500210 Specification 1 Test/box Detection principle Immunochromatographic assay Specimens SalivaIntended Use StrongStep System Device for SARS-CoV-2Antigen Rapid Test employs immunochromatography technobgy to detect the SARS-CoV-2 nucleocapsid antigen in human saliva. This test is single use only and intended for self-testing. It is recommended to use this test within 7 days of symptom onset. lt is supported by the dinical performance assessment. -

SARS-CoV-2 Antigen Rapid Test(nasal)
REF 500200 Specification 1 Tests/Box ;5 Tests/box ; 20 Tests/box Detection principle Immunochromatographic assay Specimens Anterior nasal swab Intended Use StrongStep® SARS-CoV-2 Antigen Rapid Test Cassette employs immunochromatography technology to detect the SARS- CoV-2 nucleocapsid antigen in human anterior nasal swab specimen. This testis single use only and intended for self-testing. It is recommanded to use this test within 5 days of symptom onset. It is supported by the clinical performance assessment. -

SARS-CoV-2 Antigen Rapid Test(Professional Use)
REF 500200 Specification 25 Tests/box Detection principle Immunochromatographic assay Specimens Anterior nasal swab Intended Use StrongStep® SARS-CoV-2 Antigen Rapid Test Cassette employs immunochromatography technology to detect the SARS- CoV-2 nucleocapsid antigen in human anterior nasal swab specimen. This testis single use only and intended for self-testing. It is recommanded to use this test within 5 days of symptom onset. It is supported by the clinical performance assessment. -
1人份抗原卡实物图唾液版1_00_副本-300x216.png)
SARS-CoV-2 Antigen Rapid Test for Saliva
REF 500230 Specification 20 Tests/Box Detection principle Immunochromatographic assay Specimens SalivaIntended Use This is a rapid immunochromatographic assay for the detection of SARS-CoV-2 virus Nucleocapsid Protein antigen in human Saliva swab collected from individuals who are suspected of COVID-19 by their healthcare provider within the first five days of the onset of symptoms. The assay is used as an aid in the diagnosis of COVID-19. -

System Device for SARS-CoV-2 & Influenza A/B Combo Antigen Rapid Test
REF 500220 Specification 20 Tests/Box Detection principle Immunochromatographic assay Specimens Nasal / Oropharyngeal swab Intended Use This is a rapid immunochromatographic assay for the detection of SARS-CoV-2 virus Nucleocapsid Protein antigen in human Nasal/Oropharyngeal swab collected from individuals who are suspected of COVID-19 by their healthcare provider within the first five days of the onset of symptoms. The assay is used as an aid in the diagnosis of COVID-19.







