Online Exporter Vibrio Cholerae O1/O139 Antigen Combo Rapid Test - Neisseria Gonorrhoeae Antigen Rapid Test – Liming Bio
Online Exporter Vibrio Cholerae O1/O139 Antigen Combo Rapid Test - Neisseria Gonorrhoeae Antigen Rapid Test – Liming Bio Detail:

The StrongStep® Neisseria gonorrhoeae Antigen rapid test is a rapid lateral-flow immunoassay for the qualitative presumptive detection of Neisseria gonorrhoeae antigen in male urethral and female cervical swab.
Benefits
Accurate
High sensitivity(97.5%) and high specificity(97.4%) according to the results of 1086 cases of clinical trials.
Rapid
Only 15 minutes required.
User-friendly
One-step procedure to detect the antigen directly.
Equipment-free
The source-limiting hospitals or clinical setting can perform this test.
Easy-to-read
Easily-interpreted by all healthcare personnel.
Storage conditions
Room temperature (2℃-30℃), or even higher(stable for 1 year at 37℃).
Specifications
Sensitivity 97.5%
Specificity 97.4%
CE marked
Kit Size=20 kits
File: Manuals/MSDS



Brochure1
Brochure 2
Instructions
MSDS
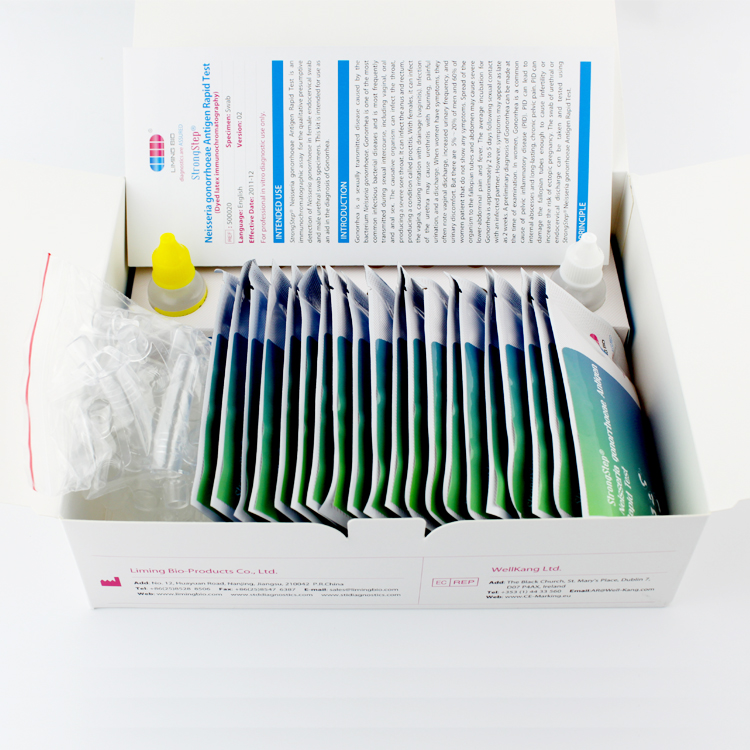
Product detail pictures:




Related Product Guide:
"Sincerity, Innovation, Rigorousness, and Efficiency" may be the persistent conception of our organization to the long-term to build together with shoppers for mutual reciprocity and mutual advantage for Online Exporter Vibrio Cholerae O1/O139 Antigen Combo Rapid Test - Neisseria Gonorrhoeae Antigen Rapid Test – Liming Bio , The product will supply to all over the world, such as: Albania, Croatia, Mexico, If you have any requests, pls email Us with your detailed demands, we will give you the most wholesale Competitive Price with the Super Quality and the Unbeatable First-class Service ! We can give you the most competitive prices and high quality, because we are much more PROFESSIONAL! So please do not hesitate to contact us.
A nice supplier in this industry, after a detail and careful discussion, we reached a consensus agreement. Hope that we cooperate smoothly.