Recently, StrongStep® SARS-CoV-2 Antigen Rapid Test (Professional Edition) from Nanjing Liming Bio-products Co., Ltd. has obtained Singapore HSA certification, Malaysia (MDA) recommended list, and is in the UK The Department of Health and Human Services (DHSC) independently evaluated and received praise.
Prior to this, StrongStep® SARS-CoV-2 antigen detection kit from Nanjing Liming Bio-products Co., Ltd. has successively obtained EU CE certification, China National Institutes for Food and Drug Control (NIFDC) registration inspection verification, entered the Rockefeller Foundation recommended list, German Federal Agency for Medicines and Medical Devices (BfArM) certification, , Guatemala certification, Indonesian FDA certification, Italian Ministry of Health certification, Philippines FDA certification, Singapore HSA certification, Ecuador certification, Brazil (ANVISA) certification, Chile certification, Argentina certification, Dominica certification, Guatemala certification and other certifications. Currently, South Africa, India, WHO's EUL, FDA's EUA, European whitelist and other certification applications are in progress.
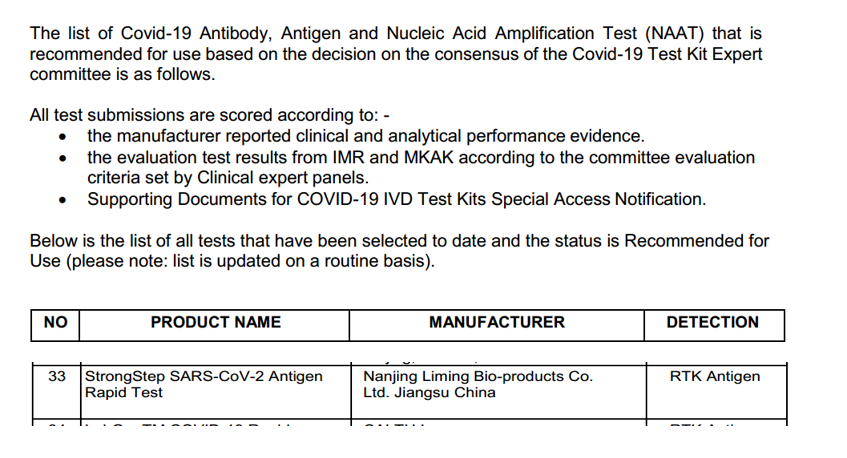
Image source: Recommended by the Malaysian Ministry of Health

Picture: Singapore HSA certification
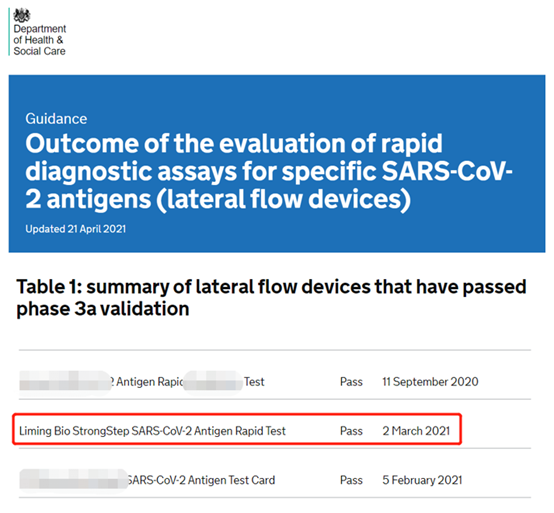
(Image source: DHSC official website of the British Department of Health and Human Services)
In 2020, the Department of Health and Human Services in the United Kingdom will strictly verify the rapid diagnostic reagents for COVID-19 entering the country to ensure that they are sufficiently accurate and reliable. There are 120 products participating in the verification process, of which only 19 products have passed the verification. After 6 months of rigorous repeated verification and verification, 200 positive specimens and 1,000 negative specimens fully proved the superior performance of Nanjing Liming Bio-products Co., Ltd.'s rapid detection reagent for COVID-19.
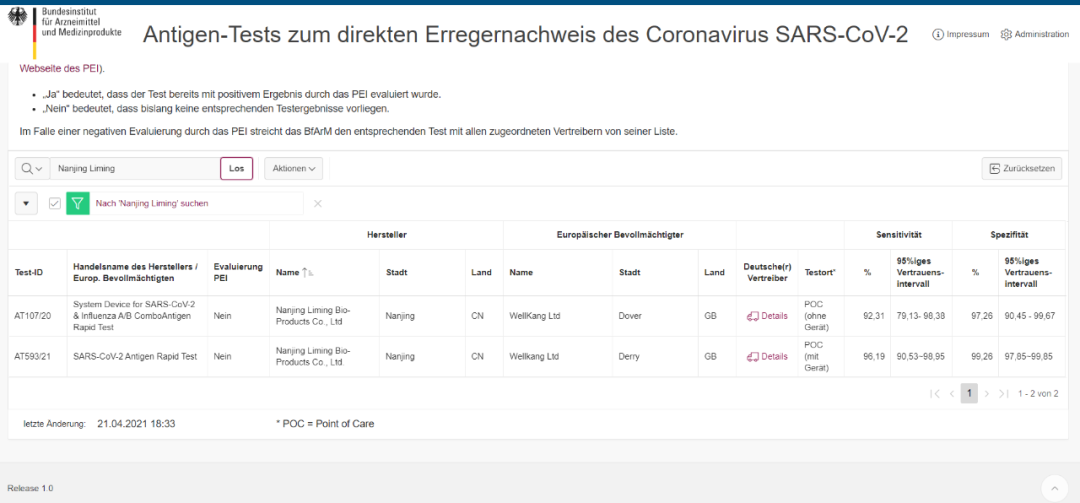
(Image source: German official Federal Agency for Medicines and Medical Devices (BfArM) website)
Official certification Test-ID: AT593/21
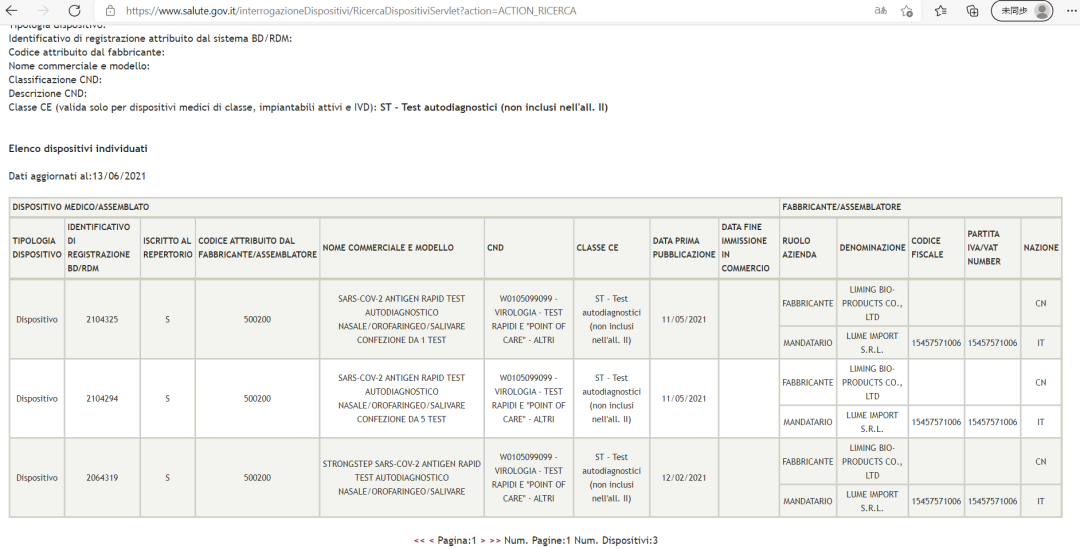
StrongStep® SARS-CoV-2 Antigen Rapid Test self-test version (Self-testing category) has been approved by the Italian Ministry of Health
Source: Official website of the Italian Ministry of Health (Ministero della Salute)

StrongStep® SARS-CoV-2 Antigen Rapid Test was praised and recommended by Italian users
The SARS-CoV-2 antigen test is fast, accurate, simple to operate, and requires low equipment and personnel. It is very suitable for the rapid investigation of suspected cases of large-scale new crown virus infection, especially for the rapid diagnosis of concentrated outbreaks. It can be used as the first line of defense for epidemic control, applied to the detection of early infections, to assist epidemic prevention and control, and control the spread of the virus.
COVID-19 will be in a long-term epidemic situation in the future, and the demand for testing will increase sharply. For different application scenarios, Nanjing Liming Bio-products Co., Ltd. has developed a variety of SARS-CoV-2 detection reagents, "SARS-CoV-2 nucleic acid detection + SARS-CoV-2 antigen detection + SARS-CoV-2 antibody detection + SARS-CoV-2 / A and B antigen triple rapid test + SARS-CoV-2 / A and B nucleic acid triple test + SARS-CoV-2 family self-inspection "The full-scenario solution meets the needs of detection and prevention at all levels in the global market. Comprehensively assist the prevention and control of the global COVID-19 epidemic and the prevention and control of respiratory diseases such as influenza.
Post time: Jun-23-2021







