Newly Arrival Vibrio Cholerae O1 Test - Screening Test for Cervical Pre-cancer and Cancer – Liming Bio
Newly Arrival Vibrio Cholerae O1 Test - Screening Test for Cervical Pre-cancer and Cancer – Liming Bio Detail:

INTENDED USE
The StrongStep® HPV 16/18 Antigen Rapid Test Device is a rapid visual immunoassay for the qualitative presumptive detection of HPV 16/18 E6&E7 oncoproteins in female cervical swab specimens. This kit is intended to be used as an aid in the diagnosis of Cervical Pre-cancer and Cancer.
INTRODUCTION
In developing countries, cervical cancer is a leading cause of cancer related death of women, due to the lack of implementation of screening tests for cervical pre-cancer and cancer. A screening test for low resource settings should be simple, rapid, and cost effective. Ideally, such a test would be informative regarding HPV oncogenic activity. Expression of both HPV E6 and E7 oncoproteins is essential for cervical cell transformation to occur. Some research results demonstrated a correlation of E6 &E7 oncoprotein positivity with both severity of cervical histopathology and risk for progression. Hence, E6&E7 oncoprotein promises to be an appropriate biomarker of HPV-mediated oncogenic activity.
PRINCIPLE
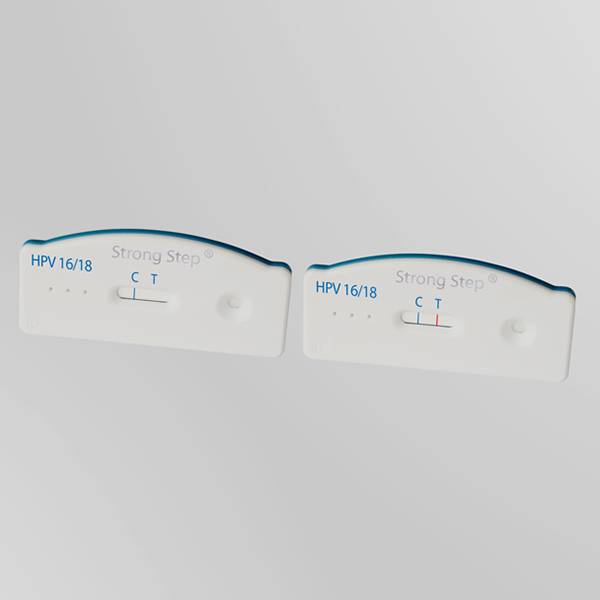
The StrongStep® HPV 16/18 Antigen Rapid Test Device has been designed to detect HPV 16/18 E6&E7 Oncoproteins through visual interpretation of color development in the internal strip. The membrane was immobilized with monoclonal anti-HPV 16/18 E6&E7 antibodies on the test region. During the test, the specimen is allowed to react with colored monoclonal anti-HPV 16/18 E6&E7 antibodies colored particals conjugates, which were precoated on the sample pad of the test. The mixture then moves on the membrane by capillary action, and interact with reagents on the membrane. If there were enough HPV 16/18 E6&E7 oncoproteins in specimens, a colored band will form at the test region of the membrane. Presence of this colored band indicates a positive result, while its absence indicates a negative result. Appearance of a colored band at the control region serves as a procedural control. This indicates that proper volume of specimen has been added and membrane wicking has occurred.
SPECIMEN COLLECTION AND STORAGE
■ The quality of specimen obtained is of extreme importance. As much as cervical epithelial cell should be collected by the swab. For cervical specimens:
■ Use only Dacron or Rayon tipped sterile swabs with plastic shafts. It is recommend to use the swab supplied by the kits manufacturer(The swab are not contained in this kit, for the ordering information, please contact the manufacture or local distributor, the cataloge number is 207000). Swabs from other suppliers have not been validated. Swabs with cotton tips or wooden shafts are not recommended.
■ Before specimen collection, remove excess mucus from the endocervical area with a separate swab or cotton ball and discard. Insert the swab into the cervix until only the bottommost fibers are exposed. Firmly rotate the swab for 15-20 seconds in one direction. Pull the swab out carefully!
■ Do not place the swab in any transport device containing medium since transport medium interferes with the assay and viability of the organisms is not required for the assay. Put the swab to the extraction tube, if the test may be run immediately. If immediate testing is not possible, the patient samples should be placed in a dry transport tube for storage or transport. The swabs may be stored for 24 hours at room temperature (15-30°C) or 1 week at 4°C or no more than 6 month at -20°C. All specimens should be allowed to reach a room temperature of 15-30°C before testing.

Cervical Pre-cancer and Cancer 1
Cervical Pre-cancer and Cancer 2
Instructions
Product detail pictures:

Related Product Guide:
We have now our possess revenue group, design staff, technical crew, QC team and package group. We now have strict excellent regulate procedures for each process. Also, all of our workers are experienced in printing subject for Newly Arrival Vibrio Cholerae O1 Test - Screening Test for Cervical Pre-cancer and Cancer – Liming Bio , The product will supply to all over the world, such as: Egypt, Hamburg, Danish, If you give us a list of merchandise you are interested in, along with makes and models, we can send you quotations. Remember to email us directly. Our goal is to establish long-term and mutually profitable business relationships with domestic and overseas clients. We look forward to receiving your reply soon.
Products and services are very good, our leader is very satisfied with this procurement, it is better than we expected,