Chinese wholesale Antibody Speed Test - Vibrio cholerae O1/O139 Antigen Combo Rapid Test – Liming Bio
Chinese wholesale Antibody Speed Test - Vibrio cholerae O1/O139 Antigen Combo Rapid Test – Liming Bio Detail:
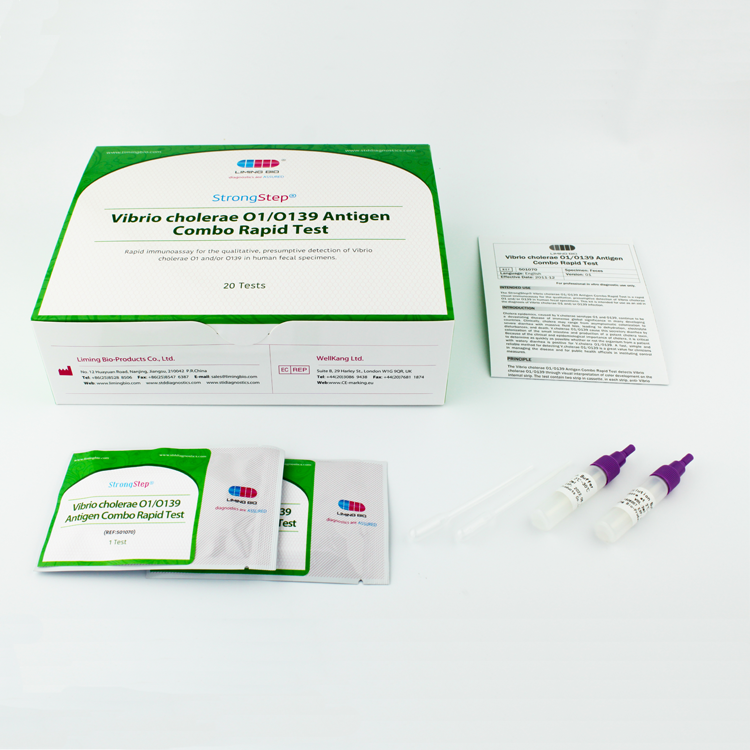

INTRODUCTION
Cholera epidemics, caused by V.cholerae serotype O1 and O139, continue to be a devastating disease of immense global significance in many developing countries. Clinically, cholera may range from asymptomatic colonization to severe diarrhea with massive fluid loss, leading to dehydration, electrolyte disturbances, and death. V.cholerae O1/O139 cause this secretory diarrhea by colonization of the small intestine and production of a potent cholera toxin, Because of the clinical and epidemiological importance of cholera, it is critical to determine as quickly as possible whether or not the organism from a patient with watery diarrhea is positive for V.cholera O1/O139. A fast, simple and reliable method for detecting V.cholerae O1/O139 is a great value for clinicians in managing the disease and for public health officials in instituting control measures.
PRINCIPLE
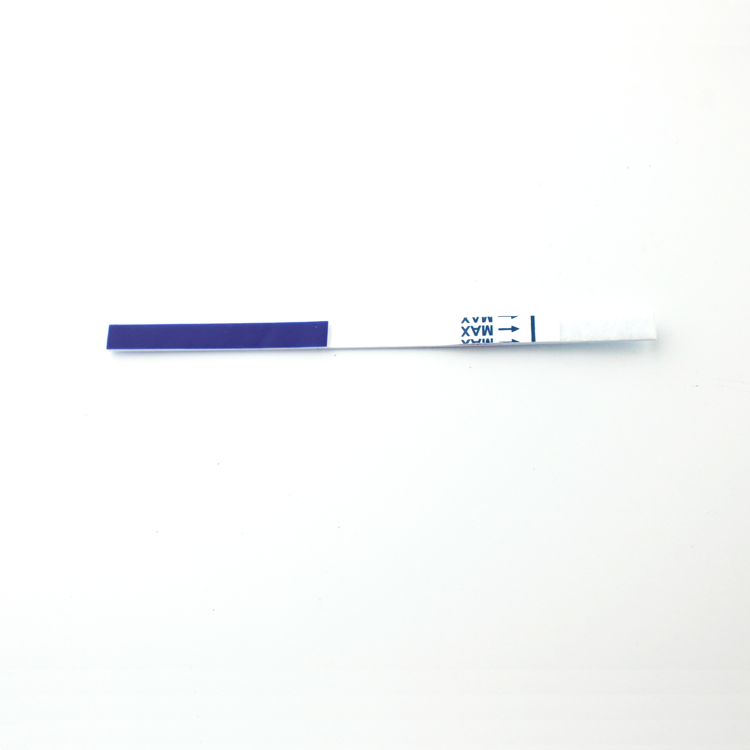
The Vibrio cholerae O1/O139 Antigen Combo Rapid Test detects Vibrio cholerae O1/O139 through visual interpretation of color development on the internal strip. The test contain two strip in cassette, in each strip, anti- Vibrio cholerae O1/O139 antibodies are immobilized on the test region of the membrane. During testing, the specimen reacts with anti- Vibrio cholerae O1/O139 antibodies conjugated to colored particles and precoated onto the conjugate pad of the test. The mixture then migrates through the membrane by capillary action and interacts with reagents on the membrane. If there is sufficient Vibrio cholerae O1/O139 in the specimen, a colored band will form at the test region of the membrane. The presence of this colored band indicates a positive result, while its absence indicates a negative result. The appearance of a colored band at the control region serves as a procedural control, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
STORAGE AND STABILITY
• The kit should be stored at 2-30°C until the expiry date printed on the sealed pouch.
• The test must remain in the sealed pouch until use.
• Do not freeze.
• Cares should be taken to protect components in this kit from contamination. Do not use if there is evidence of microbial contamination or precipitation. Biological contamination of dispensing equipments, containers or reagents can
lead to false results.
SPECIMEN COLLECTION AND STORAGE
• The Vibrio cholerae O1/O139 Antigen Combo Rapid Test is intended for use with human fecal specimens only.
• Perform testing immediately after specimen collection. Do not leave specimens at room temperature for prolonged periods. Specimens may be stored at 2-8°C for up to 72 hours.
• Bring specimens to room temperature prior to testing.
• If specimens are to be shipped, pack them in compliance with all applicable regulations for transportation of etiological agents
Product detail pictures:




Related Product Guide:
Quality First,and Customer Supreme is our guideline to provide the best service to our customers.Nowadays, we are trying our best to become one of the best exporters in our field to meet customers more need for Chinese wholesale Antibody Speed Test - Vibrio cholerae O1/O139 Antigen Combo Rapid Test – Liming Bio , The product will supply to all over the world, such as: Naples, Mauritania, US, we always keep our credit and mutual benefit to our client, insist our high quality service to moving our clients. always welcome the our friends and clients to come and visit our company and guid our business, if you are interested in our products, you can also submit your purchase information online, and we will contact you immediately, we keep our highly sincere cooperation and wish everything in your side are all well.
We have worked with many companies, but this time is the best,detailed explanation, timely delivery and quality qualified, nice!