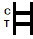2020 High quality Personal Rapid Test Device - Fetal Fibronectin Rapid Test Device – Liming Bio
2020 High quality Personal Rapid Test Device - Fetal Fibronectin Rapid Test Device – Liming Bio Detail:
|
Fetal Fibronectin Rapid Test Device |
||
| 500160 | Specimen: Swab | |
| Language: English | Version: 01 | |
| Effective Date: 2015-05 | ||
| For professional in vitro diagnostic use only. | ||
NTENDED USE
The StrongStep® PROM test is a visually interpreted immunochromatographic test intended to be used for the qualitative detection of fetal fibronectin in cervicovaginal secretions. The presence of fetal fibronectin in cervicovaginal secretions between 22 weeks, 0 days and 34 weeks, 6 days of gestation is associated with elevated risk of preterm delivery.
TRODUCTION
Preterm delivery, defined by the American College of Obstetricians and Gynecologists as delivery prior to the 37th week of gestation, is responsible for the majority of non-chromosomal perinatal morbidity and mortality. Symptoms of threatened preterm delivery include uterine contractions, change of vaginal discharge,vaginal bleeding, backache, abdominal discomfort, pelvic pressure, and cramping. Diagnostic modalities for identification of threatened preterm delivery include uterine activity monitoring and performance of a digital cervical examination, which allows estimation of cervical dimensions. These methods have been shown to be limited, as minimal cervical dilatation (< 3 centimeters) and uterine activity occur normally and are not necessarily diagnostic of imminent preterm delivery. While several serum biochemical markers have been evaluated, none have been widely accepted for practical clinical use.
Fetal fibronectin (fFN), an isoform of fibronectin, is a complex adhesive glycoprotein with a molecular weight of approximately 500,000 daltons. Matsuura and co-workers have described a monoclonal antibody called FDC-6, which specifically recognizes III-CS, the region defining the fetal isoform of fibronectin. Immunohistochemical studies of placentae have shown that fFN is confined to the extracellular matrix of the region defining the junction of the maternal and fetal units within the uterus.
Fetal fibronectin can be detected in cervicovaginal secretions of women throughout pregnancy by use of a monoclonal antibodybased immunoassay. Fetal fibronectin is elevated in cervicovaginal secretions during early pregnancy but is diminished from 22 to 35 weeks in normal pregnancies. The significance of its presence in the vagina during the early weeks of pregnancy is not understood. However, it may simply reflect the normal growth of the extravillous trophoblast population and the placenta. Detection of fFN in cervicovaginal secretions between 22 weeks, 0 days and 34 weeks, 6 days gestation is reported to be associated with preterm delivery in symptomatic and between 22 weeks, 0 days and 30 weeks, 6 days in asymptomatic pregnant women.
PRINCIPLE
The StrongStep® fFN Test uses color immunochromatographic, capillary flow technology. The test procedure requires the solubilization of fFN from a vaginal swab by mixing the swab in Sample Buffer. Then the mixed sample buffer is added to the test cassette sample well and the mixture migrates along the membrane surface. If fFN is present in the sample, it will form a complex with the primary anti- fFN antibody conjugated to colored particles. The complex will then be bound by a second anti- fFN antibody coated on the nitrocellulose membrane. The appearance of a visible test line along with the control line will indicate a positive result.
KIT COMPONENTS
|
20 Individually packed test devices |
Each device contains a strip with colored conjugates and reactive reagents pre-coated at the corresponding regions. |
|
2 Extraction Buffer vial |
0.1 M Phosphate buffered saline (PBS) and 0.02% sodium azide. |
| 1 Positive control swab (on request only) |
Contain fFN and sodium azide. For External control. |
| 1 Negative control swab (on request only) |
Not contain fFN. For external control. |
|
20 Extraction tubes |
For specimens preparation use. |
|
1 Workstation |
Place for holding buffer vials and tubes. |
|
1 Package insert |
For operation instruction. |
MATERIALS REQUIRED BUT NOT PROVIDED
| Timer | For timing use. |
PRECAUTIONS
■ For professional in vitro diagnostic use only.
■ Do not use after expiration date indicated on the package. Do not use the test if its foil pouch is damaged. Do not reuse tests.
■ This kit contains products of animal origin. Certified knowledge of the origin and/or sanitary state of the animals does not totally guarantee the absence of transmissible pathogenic agents. It is therefore, recommended that these products be treated as potentially infectious, and handled observing the usual safety precautions (do not ingest or inhale).
■ Avoid cross-contamination of specimens by using a new specimen collection container for each specimen obtained.
■ Read the entire procedure carefully prior to performing any tests.
■ Do not eat, drink or smoke in the area where the specimens and kits are handled. Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout the procedure and follow the standard procedures for proper disposal of specimens. Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are assayed.
■ Do not interchange or mix reagents from different lots. Do not mix solution bottle caps.
■ Humidity and temperature can adversely affect results.
■ When the assay procedure is completed, dispose the swabs carefully after autoclaving them at 121°C for at least 20 minutes. Alternatively, they can be treated with 0.5% sodium hypochloride (or house-hold bleach) for one hour before disposal. The used testing materials should be discarded in accordance with local, state and/or federal regulations.
■ Do not use cytology brushes with pregnant patients.
STORAGE AND STABILITY
■ The kit should be stored at 2-30°C until the expiry date printed on the sealed pouch.
■ The test must remain in the sealed pouch until use.
■ Do not freeze.
■ Cares should be taken to protect components in this kit from contamination. Do not use if there is evidence of microbial contamination or precipitation. Biological contamination of dispensing equipments, containers or reagents can lead to false results.
PECIMEN COLLECTION AND STORAGE
■ Use only Dacron or Rayon tipped sterile swabs with plastic shafts. It is recommend to use the swab supplied by the kits manufacturer(The swabs are not contained in this kit, for the ordering information, please contact the manufacturer or local distributor, the cataloge number is 207000). Swabs from other suppliers have not been validated. Swabs with cotton tips or wooden shafts are not recommended.
■ Cervicovaginal secretions are obtained from the posterior fornix of the vagina. The collection process is intended to be gentle. Vigorous or forceful collection, common for microbiological cultures, is not required. During a speculum examination, prior to any examination or manipulation of the cervix or the vaginal tract, lightly rotate the applicator tip across the posterior fornix of the vagina for approximately 10 seconds to absorb cervicovaginal secretions. Subsequent attempts to saturate the applicator tip may invalidate the test. Remove the applicator and perform the test as directed below.
■ Put the swab to the extraction tube, if the test may be run immediately. If immediate testing is not possible, the patient samples should be placed in a dry transport tube for storage or transport. The swabs may be stored for 24 hours at room temperature (15-30°C) or 1 week at 4°C or no more than 6 month at -20°C. All specimens should be allowed to reach a room temperature of 15-30°C before testing.
PROCEDURE
Bring tests, specimens, buffer and/or controls to room temperature (15-30°C) before use.
■ Place a clean Extraction tube in the designated area of the workstation. Add 1ml of Extraction Buffer to the extraction tube.
■ Put the specimen swab into the tube. Vigorously mix the solution by rotating the swab forcefully against the side of the tube for least ten times (while submerged). Best results are obtained when the specimen is vigorously mixed in the solution.
■ Squeeze out as much liquid as possible from the swab by pinching the side of the flexible extraction tube as the swab is removed. At least 1/2 of the sample buffer solution must remain in the tube for adequate capillary migration to occur. Put the cap onto the extracted tube.
Discard the swab in a suitable biohazardous waste container.
■ The specimens extracted can retain at room temperature for 60 minutes without affecting the result of the test.
■ Remove the test from its sealed pouch, and place it on a clean, level surface. Label the device with patient or control identification. To obtain a best result, the assay should be performed within one hour.
■ Add 3 drops (approximately 100 µl) of extracted sample from the Extraction Tube to the sample well on the test cassette.
Avoid trapping air bubbles in the specimen well (S), and do not drop any solution in observation window.
As the test begins to work, you will see color move across the membrane.
■ Wait for the colored band(s) to appear. The result should be read at 5 minutes. Do not interpret the result after 5 minutes.
Discard used test tubes and Test Cassettes in suitable biohazardous waste container.
NTERPRETATION OF RESULTS
|
POSITIVE RESULT:
|
Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T). |
|
NEGATIVE RESULT:
|
Only one colored band appears in the control region (C). No apparent colored band appears in the test region (T). |
|
INVALID RESULT:
|
Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor. |
NOTE:
1. The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. But the substances level can not be determined by this qualitative test.
2. Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
QUALITY CONTROL
■ Internal procedural controls are included in the test. A colored band appearing in the control region (C) is considered as an internal positive procedural control. It confirms sufficient specimen volume and correct procedural technique.
■ External procedural controls may provided(on request only) in the kits to ensure that the tests are functioning properly. Also, the Controls may be used to demonstrate proper performance by the test operator. To perform a positive or negative control test, complete the steps in the Test Procedure section treating the control swab in the same manner as a specimen swab.
LIMITATIONS OF THE TEST
1. This assay can only be used for the qualitative detection of fetal fibronectin in cervicovaginal secretions.
2. Test results should always be used in conjunction with other clinical and laboratory data for patient management.
3. Specimens should be obtained prior to digital examination or manipulation of the cervix. Manipulations of the cervix may lead to false positive results.
4. Specimens should not be collected if the patient has had sexual intercourse within 24 hours to eliminate false positive results.
5. Patients with suspected or known placental abruption, placenta previa, or moderate or gross vaginal bleeding should not be tested.
6. Patients with cerclage should not be tested.
7. The performance characteristics of the StrongStep® fFN test are based on studies in women with singleton gestations. Performance has not been verified on patients with multiple gestations, e.g., twins.
8. The StrongStep® fFN test is not intended to be performed in the presence of rupture of amniotic membranes and rupture of amniotic membranes should be ruled out prior to conducting the test.
PERFORMANCE CHARACTERISTICS
Table: StrongStep® fFN Test vs. Another brand fFN Test
|
Relative Sensitivity: 97.96% (89.13%-99.95%)* Relative Specificity: 98.73% (95.50%-99.85%)* Overall Agreement: 98.55% (95.82%-99.70%)* *95% Confidence Interval |
|
Another brand |
|
||
|
+ |
- |
Total |
|||
|
StrongStep® fFn Test |
+ |
48 |
2 |
50 |
|
|
- |
1 |
156 |
157 |
||
|
|
49 |
158 |
207 |
||
Analytic sensitivity
The lowest detectable amount of fFN in the extracted sample is 50μg/L.
Among symptomatic women, elevated levels (≥ 0.050 μg/mL) (1 x 10-7 mmol/L) of fFN between 24 weeks, 0 days and 34 weeks, 6 days indicate increased risk of delivery in ≤ 7 or ≤ 14 days from sample collection. Among asymptomatic women, elevated levels of fFN between 22 weeks, 0 days and 30 weeks, 6 days indicate increased risk of delivery in ≤ 34 weeks, 6 days of gestation. The cutoff of 50 μg/L fFN was established in a multicenter study conducted to evaluate the association between fetal fibronectin expression during pregnancy and preterm delivery.
Interfering Substances
Care must be taken not to contaminate the applicator or cervicovaginal secretions with lubricants, soaps, disinfectants, or creams. Lubricants or creams may physically interfere with absorption of the specimen onto the applicator. Soaps or disinfectants may interfere with the antibody-antigen reaction.
Potential interfering substances were tested at concentrations that might be reasonably found in cervicovaginal secretions. The following substances did not interfere in the assay when tested at the levels indicated.
| Substance | Concentration | Substance | Concentration |
| Ampicillin | 1.47 mg/mL | Prostaglandin F2 | a 0.033 mg/mL |
| Erythromycin | 0.272 mg/mL | Prostaglandin E2 | 0.033 mg/mL |
| Maternal Urine 3rd Trimester | 5% (vol) | MonistatR (miconazole) | 0.5 mg/mL |
| Oxytocin | 10 IU/mL | Indigo Carmine | 0.232 mg/mL |
| Terbutaline | 3.59 mg/mL | Gentamicin | 0.849 mg/mL |
| Dexamethasone | 2.50 mg/mL | BetadineR Gel | 10 mg/mL |
| MgSO4•7H2O | 1.49 mg/mL | BetadineR Cleanser | 10 mg/mL |
| Ritodrine | 0.33 mg/mL | K-YR Jelly | 62.5 mg/mL |
| DermicidolR 2000 | 25.73 mg/mL |
LITERATURE REFERENCES
1. American College of Obstetricians and Gynecologists. Preterm Labor. Technical Bulletin, Number 133, October, 1989.
2. Creasy RK, Resnick R. Maternal and Fetal Medicine: Principles and Practice. Philadelphia: W.B. Saunders; 1989.
3. Creasy RK, Merkatz IR. Prevention of preterm birth: clinical opinion. Obstet Gynecol 1990;76(Suppl 1):2S–4S.
4. Morrison JC. Preterm birth: a puzzle worth solving. Obstet Gynecol 1990;76(Suppl 1):5S-12S.
5. Lockwood CJ, Senyei AE, Dische MR, Casal DC, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. New Engl J Med 1991;325:669–74.
GLOSSARY OF SYMBOLS
|
|
Catalog number |
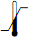 |
Temperature limitation |
 |
Consult instructions for use |
|
Batch code |
 |
In vitro diagnostic medical device |
 |
Use by |
 |
Manufacturer |
 |
Contains sufficient for <n> tests |
 |
Do not reuse |
 |
Authorized representative in the European Community |
 |
CE marked according to IVD Medical Devices Directive 98/79/EC |
||
Liming Bio-Products Co., Ltd.
No. 12 Huayuan Road,Nanjing, Jiangsu, 210042 P.R. China.
Tel: (0086)25 85476723 Fax: (0086)25 85476387
E-mail: sales@limingbio.com
Website: www.limingbio.com
www.stddiagnostics.com
www.stidiagnostics.com
WellKang Ltd.(www.CE-marking.eu) Tel: +44(20)79934346
29 Harley St., London WIG 9QR,UK Fax: +44(20)76811874




StrongStep® Fetal Fibronectin Rapid Test Device

Preterm delivery, defined by the American College of Obstetricians and Gynecologists as delivery prior to the 37th week of gestation, is responsible for the majority of non-chromosomal perinatal morbidity and mortality. Symptoms of threatened preterm delivery include uterine contractions, change of vaginal discharge,vaginal bleeding, backache, abdominal discomfort, pelvic pressure, and cramping. Diagnostic modalities for identification of threatened preterm delivery include uterine activity monitoring and performance of a digital cervical examination, which allows estimation of cervical dimensions.
StrongStep® Fetal Fibronectin Rapid Test is a visually interpreted immunochromatographic test intended to be used for the qualitative detection of fetal fibronectin in cervicovaginal secretions with the following characteristics:
User friendly: one-step procedure in qualitative testing
Rapid: only 10 minutes required during the same patient visiting
Equipment-free: the source-limiting hospitals or clinical setting can perform this test
Delivered: room temperature (2℃-30℃)
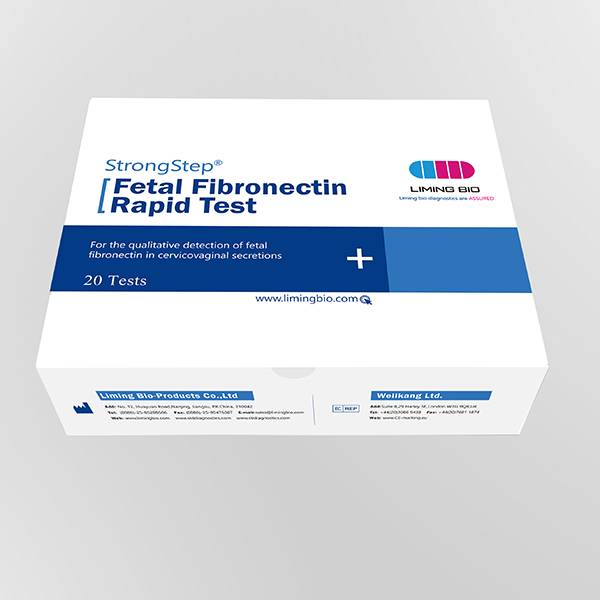
Product detail pictures:



Related Product Guide:
High quality Very first,and Consumer Supreme is our guideline to offer the most beneficial service to our consumers.At present, we're attempting our greatest to be among the top exporters in our area to fulfill buyers far more need to have for 2020 High quality Personal Rapid Test Device - Fetal Fibronectin Rapid Test Device – Liming Bio , The product will supply to all over the world, such as: Slovenia, Kuala Lumpur, Bolivia, With the aim of "compete with good quality and develop with creativity" and the service principle of "take customers' demand as orientation", we will earnestly provide qualified products and solutions and good service for domestic and international customers.
Product quality is good, quality assurance system is complete, every link can inquire and solve the problem timely!