2020 Good Quality Sars-Cov-2 Pcr - StrongStep System Device for SARS-CoV-2 Antigen Rapid Test – Liming Bio
2020 Good Quality Sars-Cov-2 Pcr - StrongStep System Device for SARS-CoV-2 Antigen Rapid Test – Liming Bio Detail:
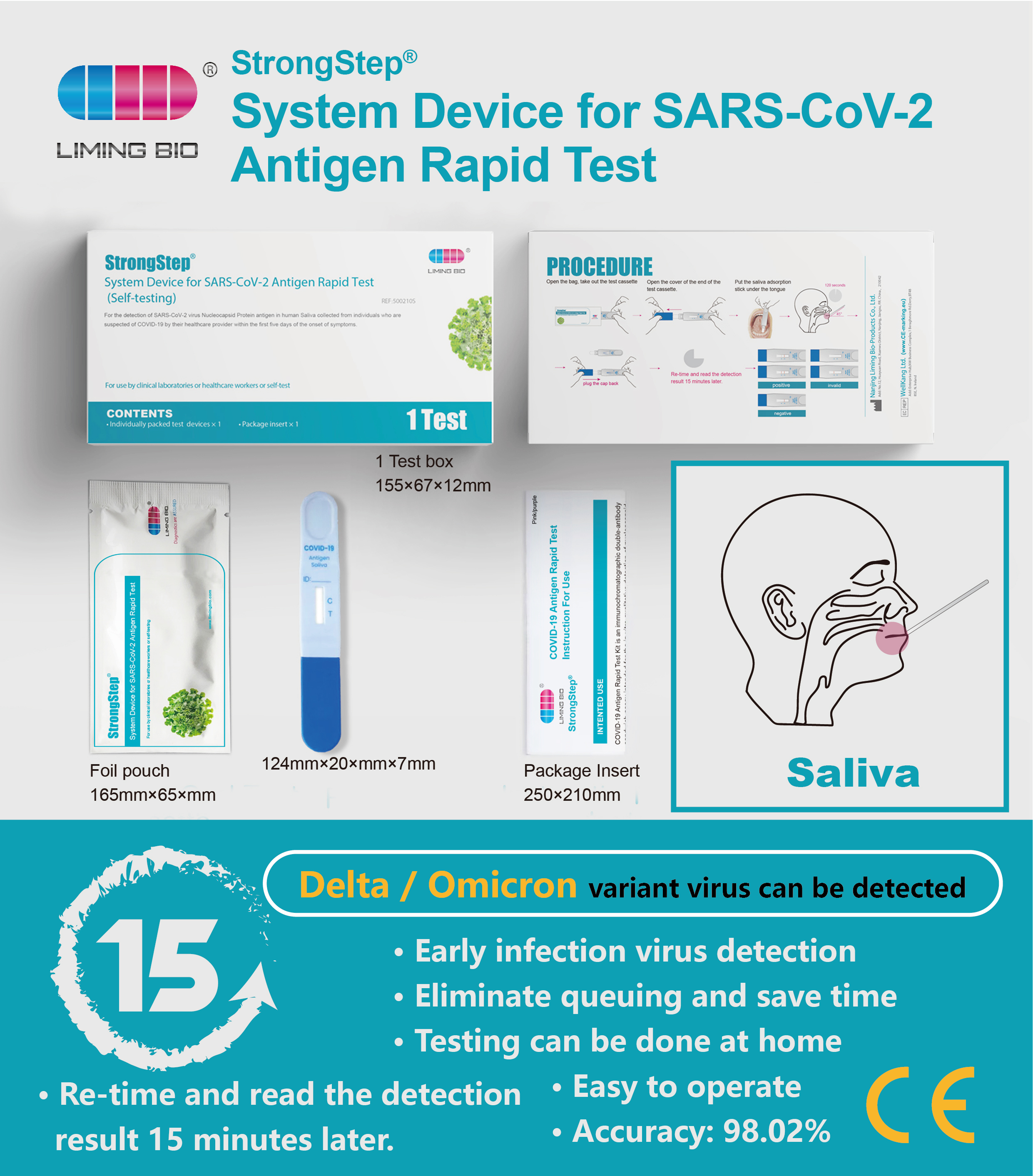
INTENDED USE
StrongStep® System Device for SARS-CoV-2 Antigen Rapid Test employs immunochromatography technology to detect the SARS- CoV-2 nucleocapsid antigen in human saliva. This test is single use only and intended for sei—testing. It is recommended to use this test within 7 days of symptom onset It is supported by the clinical performance assessment.
INTRODUCTION
The novel coronaviruses belong to the 0 genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
PRINCIPLE
The StrongStep® System Device for SARS-CoV-2 Antigen Test employs immunochromatographic test, this kit collects saliva samples from the saliva adsorption stick at the front of the test card, and the saliva samples move forward under capillary action. If the sample contains a SARS-CoV-2 N protein antigen.it is identified and bound by antibodies labeled on the latex surface to form an immune complex. When the formed immune complex migrates to the nitric acid fiber membrane detection line, to identify the packed antibodies and form a fuchsia detection line (T-lins), showing SARS-Cov-2 antigen positive; if the T-line does not show color, it is a negative result. Another line on the nitric acid fiber membrane is packed with Streptavidin antibodies as a quality control line (C line),to indicate an effective testing process.
PRECAUTIONS
•This kit is for IN VITRO diagnostic use only.
•This kit can be administered by medical or non-medical personnel by following the operating instructions.
•Read the instructions carefully before performing the test.
•This product does not contain any human source materials.
•Do not use kit contents after the expiration date.
•Handle all specimens as potentially infectious.
•Do not pipette reagent by mouth and no smoking or eating while performing assays.
•Wear gloves during the whole procedure.
STORAGE AND STABILITY
The sealed pouches in the test kit may be stored between 2-30°C for the duration of the shelf life as indicated on the pouch.
SPECIMEN COLLECTION AND STORAGE
Best saliva specimen should be collect in the morning after just wake up. Do not eat or drink anything for 30 minutes prior to collecting your saliva sample. Do it before having coffee, eating breakfast, or brushing your teeth — or wait until you haven’t consumed anything in the prior 30 minutes.
PROCEDURE
Bring tests to room temperature (15-30°C) before use.
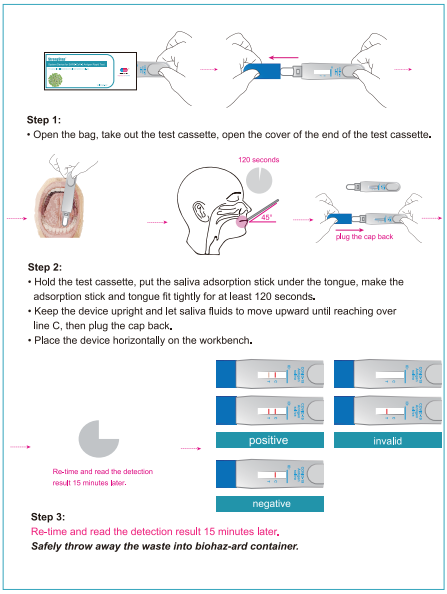
Step 1:
Open the bag, take out the test device, open the cover of the end of the test device.
Step 2:
•Hold the lest cassette, put the saliva adsorption stick under the tongue, make the adsorption stick and tongue fit tightly for at least 120 seconds.
•Keep the device upright and let saliva fluids to move upward until reaching over line C, then plug the cap back.
•Place the device horizontally on the workbench.
Step 3:
Re-time and read the detection resuh 15 minutes later.
Safely throw away the waste into biohaz-ard container.
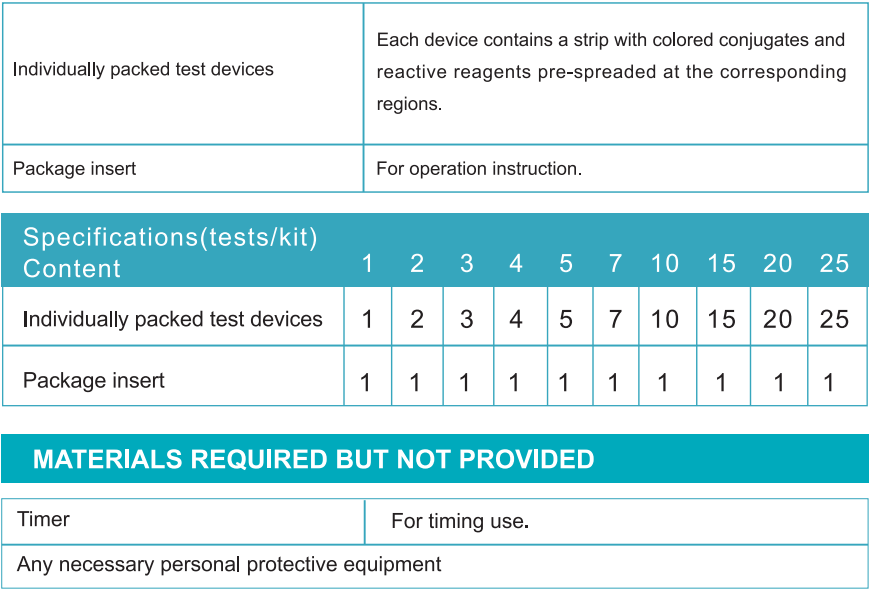
QUALITY CONTROL
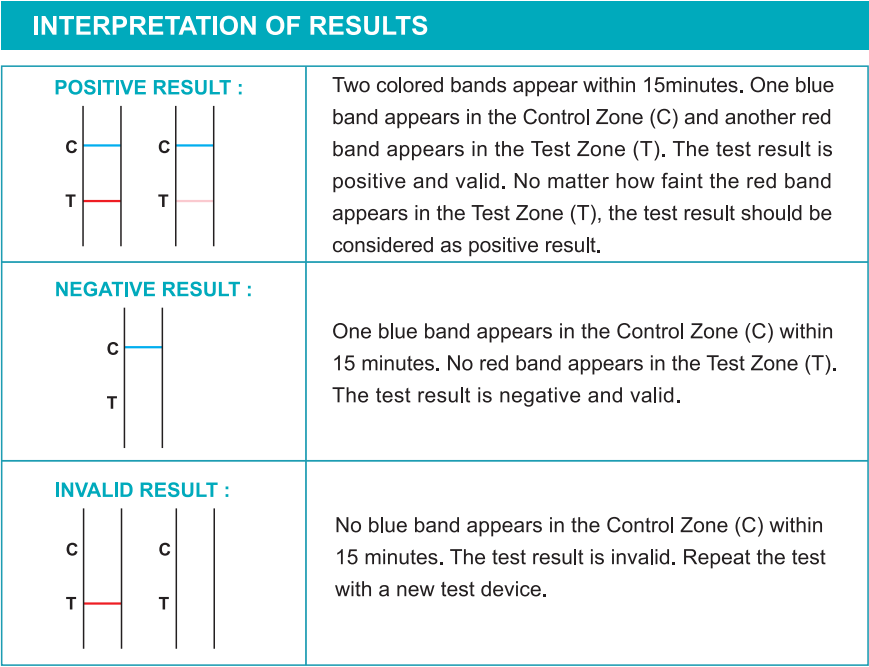
Internal procedural controls are included in the test. A blue band appearing in the control region (C) is considered as an internal procedural control. It confirms sufficient specimen volume and correct procedural technique.
LIMITATIONSOF THE TEST
1. The kit is intended to use for the qualitative detection of SARS-CoV-2 antigens from saliva.
2. This test detects both viable (live) and non-viable SARS-CoV-2. Test performance depends on the amount of virus (antigen) in the sample and may or may not correlate with viral culture results performed on the same sample.
3. A negative test result may occur if the level of antigen in a sample is below the detection limit of the test or if the sample was collected or transported improperly.
4. Failure to follow the Test Procedure may adversely affect test performance and/or invalidate the test result.
5. The kit is for presumptive screening only. Negative results do not rule out SARS-CoV-2 infection and the person not being infectious. If symptoms are present, seek immediate further testing.
6. Test results must be correlated with the clinical history, epidemiological data, and other data available to the clinician evaluating the patient.
7. Positive test results do not rule out co-infections with other pathogens and cannot necessarily determine whether a person is infectious.
8. Negative test results are not intended to rule in other non-SARS viral or bacterial infections.
9. Negative results from patients with symptom , should be treated as presumptive and confirmed with an local FDA authorized molecular assay, if necessary, for clinical management, including infection control.
10. Specimen stability recommendations are based upon stability data from influenza testing and performance may be different with SARS-CoV-2. Users should test specimens as quickly as possible after specimen collection.
11. The sensitivity for RT-PCR assay in diagnosis of COVID-19 is only 50%-80% due to poor sample quality or disease time point at the recoverd phase,etc.SARS-CoV-2 Antigen Rapid Test Device’s sensitivity is theoretically lower because of its methodology.
12. Positive and negative predictive values are highly dependent on prevalence rates.
Positive test results are more likely to represent false positive results during periods of little / no SARS-CoV-2 activity when disease prevalence is low.False negative test results are more likely when prevalence of disease caused by SARS-CoV-2 is high.
13. Monoclonal antibodies may fail to detect, or detect with less sensitivity,SARS-CoV-2 influenza viruses that have undergone minor amino acid changes in the target epitope region.
14. The performance of this test has not been evaluated for use in patients without signs and symptoms of respiratory infection and performance may differ in asymptomatic individuals.
15. The amount of antigen in a sample may decrease as the duration of illness increases.
Specimens collected after day 7 of illness are more likely to be negative compared to a RT-PCR assay.
Sensitivity of the test after the seven days of the onset of symptoms has been known to decrease as compared to a RT-PCR assay.
16. It is not recommend to use Virus Transportation media(VTM) specimen in this test, if customers insist to use this sample type, customers should validate themselves.
17. Frequent testing is necessary to increase the sensitivity of diagnosis of COVID-19.
18. No drop off in sensitivity when compared with the wild type with respect to the following variants -B.1.1.7; B.1.351; B.1.2; B.1.1.28; B.1.617; B.1.1.529.
19. Positive results indicate that viral antigens were detected in the sample taken, please self-quarantine and inform your family doctor promptly and/or your local health department in accordance with State requirements.
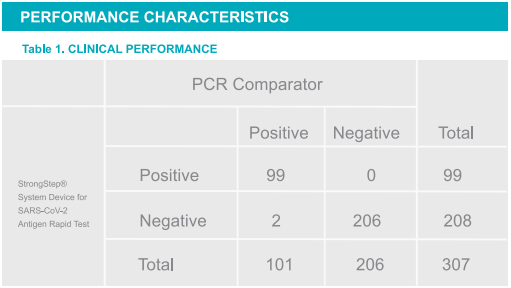
Positive Percent Agreement: (PPA)= 98.02%(93.03%~99.76%)*
Negative Percent Agreement: (NPA)= 100% (98.23%~100%)*
Total coincidence rate = 98.76%
*95%Confidence Interval
ANALYTICAL PERFORMANCE
a) Limit of Detection (LoD):
The Limit of Detection (LoD) of the test was determined using limiting dilutions of inactivated SARS-CoV-2. It is a preparation of SARS-Related Coronavirus-2 (SARS-CoV-2), isolating in China CDC, that has been inactivated by β-propiolactone. The material was supplied frozen at a concentration of TCID50 of 5.00 x105/mL.
To determine the SARS-CoV-2 to reflect the assay when using direct saliva. In this study approximately 50μL of the virus dilution was spiked with the saliva negative sample.
The LoD was determined in three steps:
1. LoD Screening
10-fold dilutions of the inactivated virus were made in negative saliva and processed for each study as described above. These dilutions were tested in triplicate. The concentration demonstrating 3 of 3 positives was chosen for LoD range finding.
2. LoD Range Finding
Five (5) doubling dilutions were made of the TCID50 of 5.00 x102/mL concentration in negative saliva processed for the study as described above. These dilutions were tested in triplicate. The concentration demonstrating 3 of 3 positives was chosen for LoD confirmation.
3. LoD Confirmation
The concentration TCID50 of 2.50 x102/mL dilution was tested for a total of twenty (20) results. At least nineteen (19) of twenty (20) results were positive.
Conclusion:
Based on this testing the concentration was confirmed as:
LoD: TCID50 2.50 x102/mL
b) Cross-Reactivity:
Cross-reactivity of the StrongStep® System Device SARS-CoV-2 Antigen Rapid Test was evaluated by testing various microorganisms (10⁶ CFU/mL), viruses (10⁵ PFU/mL) and negative matrixes that may potentially cross-react with the StrongStep® System Device SARS-CoV-2 Antigen Rapid Test.
Each organism and virus were tested in triplicate. Based on the data generated by this study, the StrongStep® System Device for SARS-CoV-2 Antigen Rapid Test does not cross-react with the organisms or viruses tested.
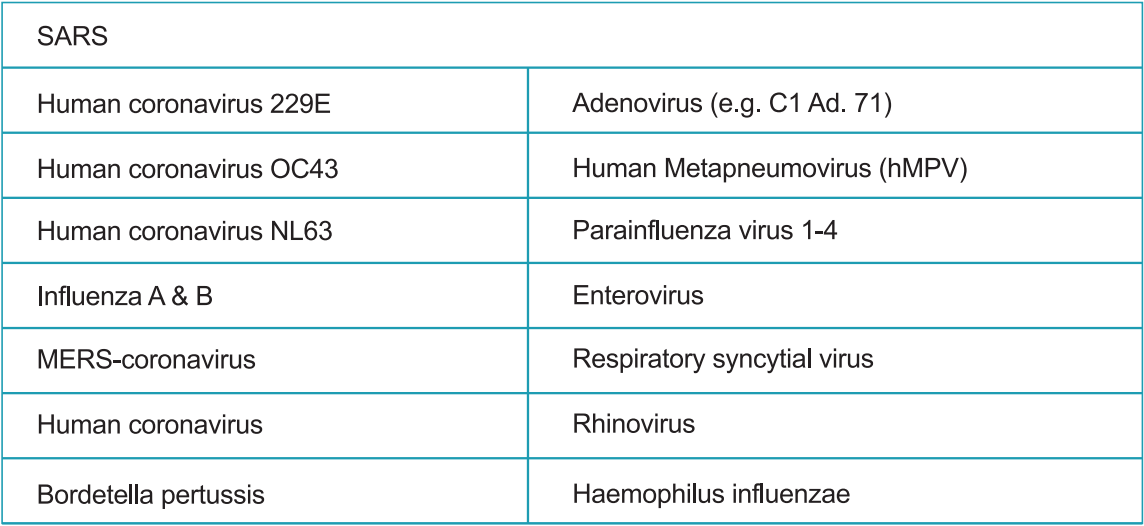
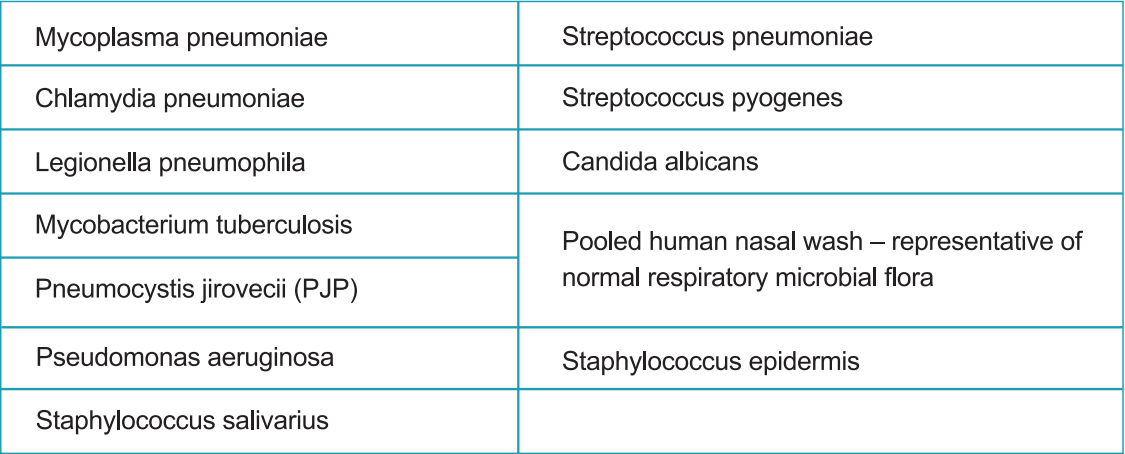
c)Interfere substance:
Potential interfering substances of the StrongStep® SARS-CoV-2 Antigen Rapid Test was evaluated by testing various substances with concentration below that may potentially interfere with the StrongStep® SARS-CoV-2 Antigen Rapid Test. Each substance were tested in triplicate. Based on the data generated by this study, the StrongStep® SARS-CoV-2 Antigen Rapid Test does not interfere with the substances tested.
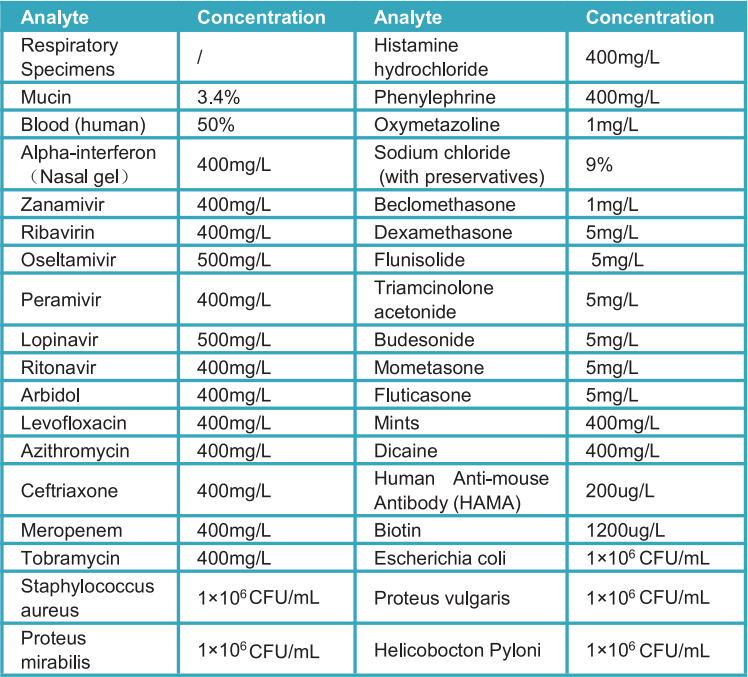
d) Hook Effect
The highest concentration of heat-inactivated SARS-CoV-2 stock available (TCID50 of 5.00 x 105/mL) was tested. There was no Hook effect detected.
Product detail pictures:


Related Product Guide:
"Quality initial, Honesty as base, Sincere support and mutual profit" is our idea, so as to build repeatedly and pursue the excellence for 2020 Good Quality Sars-Cov-2 Pcr - StrongStep System Device for SARS-CoV-2 Antigen Rapid Test – Liming Bio , The product will supply to all over the world, such as: St. Petersburg, Bahrain, Berlin, We have got constantly insisted on the evolution of solutions, spent good funds and human resource in technological upgrading, and facilitate production improvement, meeting the wants of prospects from all countries and regions.
The company comply with the contract strict, a very reputable manufacturers, worthy a long-term cooperation.