2020 Good Quality Sars-Cov-2 Pcr - SARS-CoV-2 Antigen Rapid Test – Liming Bio
2020 Good Quality Sars-Cov-2 Pcr - SARS-CoV-2 Antigen Rapid Test – Liming Bio Detail:
INTENDED USE
TheStrongStep® SARS-CoV-2 Antigen Rapid Test is a rapid immunochromatographic assay for the detection of COVID-19 antigen to SARS-CoV-2 virus in human Throat/Nasopharyngeal swab. The assay is used asan aid in the diagnosis of COVID-19.
INTRODUCTION
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose,sore throat, myalgia and diarrhea are found in a few cases.
PRINCIPLE
The StrongStep® SARS-CoV-2 Antigen Test employs chromatographic lateral flow test device in a cassette format. Latex conjugated antibody (Latex-Ab) corresponding to SARS- CoV-2 are dry-immobilized at the end of nitrocellulose membrane strip. SARS-CoV-2 antibodies are bond at the Test Zone (T) and Biotin-BSA are bond at the Control Zone (C). When the sample is added, it migrates by capillary diffusion rehydrating the latex conjugate. If present in sample, SARS-CoV- 2 antigens will bind with the least conjugated antibodies forming particles. These particles will continue to migrate along the strip until the Test Zone (T) where they are captured by SARS-CoV-2 antibodies generating a visible red line. If there are no anti- SARS-CoV-2 antigens in sample, no red line is formed in the Test Zone(T). The streptavidin conjugate will continue to migrate alone until it is captured in the Control Zone(C) by the Biotin-BSA aggregating in a line, which indicates the validity of the test.
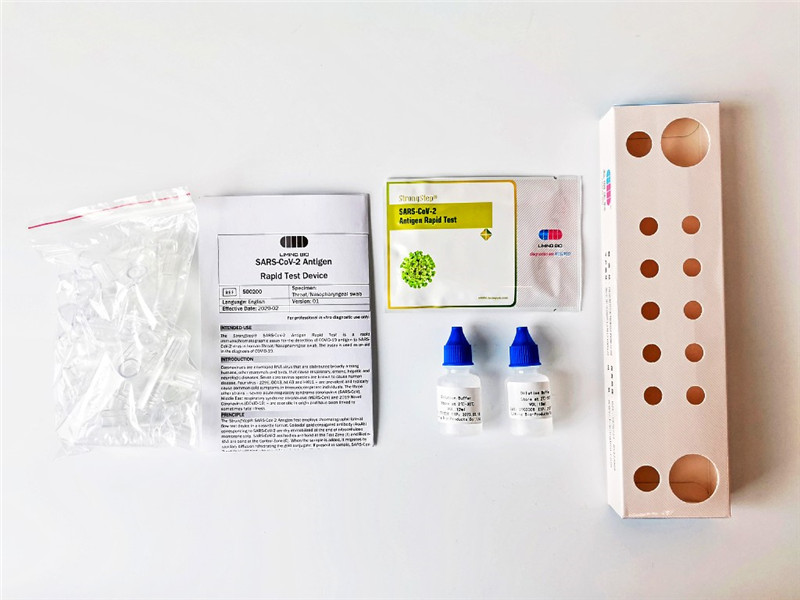
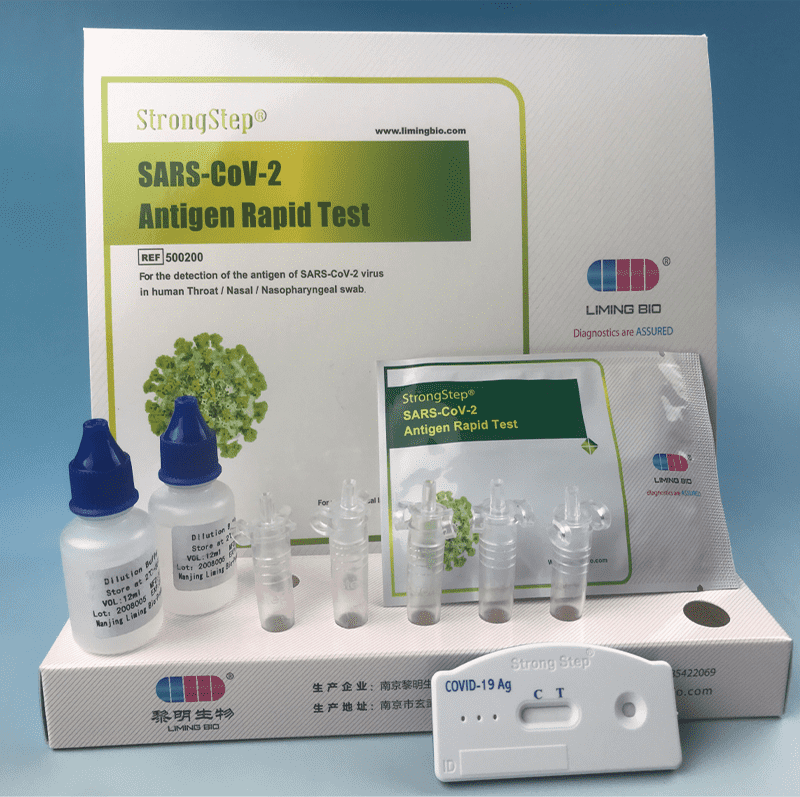
KIT COMPONENTS
|
20 Individually packed test devices |
Each device contains a strip with colored conjugates and reactive reagents pre-spreaded at the corresponding reqions. |
|
2 Extraction Buffer vials |
0.1 M Phosphate buffered saline (P8S) and0.02% sodium azide. |
|
20 Extraction tubes |
For specimens preparation use. |
|
1 Workstation |
Place for holding buffer vials and tubes. |
|
1 Package insert |
For operation instruction. |
MATERIALS REQUIRED BUT NOT PROVIDED
| Timer | For timing use. |
| Throat/Nasopharyngeal swab | For specimen collection |
PRECAUTIONS
This kit is for IN VITRO diagnostic use only.
This kit is For Medical Professional Use Only.
Read the instructions carefully before performing the test.
This product does not contain any human source materials.
Do not use kit contents after the expiration date.
Handle all specimens as potentially infectious.
Follow standard Lab procedure and biosafety guidelines for handling and disposal of potentially infective material. When the assay procedure is complete, dispose specimens after autoclaving them at 121℃ for at least 20 minutes. Alternatively, they can be treated with0.5% Sodium Hypochlorite four hours before disposal.
Do not pipette reagent by mouth and no smoking or eating while performing assays.
Wear gloves during the whole procedure.
STORAGE AND STABILITY
The sealed pouches in the test kit may be stored between 2- 30℃ for the duration of the shelf life as indicated on the pouch.
SPECIMEN COLLECTION AND STORAGE
Nasopharyngeal Swab Sample: It is important to obtain as much secretion as possible. Therefore, to collect a Nasopharyngeal Swab sample, carefully insert the sterile Swab into the nostril that presents the most secretions under visual inspection. Keep the Swab near the septum floor of the nose while gently pushing the Swab into the posterior nasopharynx. Rotate the Swab several times. Throat swab: Depress the tongue with a tongue blade or spoon. When swabbing the throat, be careful not to touch the tongue, sides or top of the mouth with the Swab. Rub the Swab on the back of the throat, on the tonsils and in any other area where there is redness, inflammation or pus. Use rayon tipped swabs to collect specimens. Do not use calcium alginate, cotton tipped or wooden shaft swabs.
It is recommended that swab specimens be processed as soon as possible after collection. Swabs can be held in any clean, dry plastic tube or sleeve up to 72 hours at room temperature (15°C to 30°C), or refrigerated (2°C to 8°C) before processing.
PROCEDURE
Bring tests, specimens, buffer and/or controls to room temperature (15-30°C) before use.
1. Place a clean Extraction tube in the designated area of the workstation. Add 10 drops of Extraction Buffer to the extraction tube.
2. Put the specimen swab into the tube. Vigorously mix the solution by rotating the swab force fully against the side of the tube for at least ten times (while submerged).Best results are obtained when the specimen is vigorously mixed in the solution. Allow the swab to soak in the Extraction Buffer for one minute prior to the next Step.
3. Squeeze out as much liquid as possible from the swab by pinching the side of the flexible extraction tube as the swab is removed. At least 1/2 of the sample buffer solution must remain in the tube for adequate capillary migration to occur. Put the cap onto the extracted tube. Discard the swab in a suitable biohazardous waste container.
4. The specimens extracted can retain at room temperature for 60 minutes without affecting the result of the test.
5. Remove the test from its sealed pouch, and place it on a clean, level surface. Label the device with patient or control identification. To obtain a best result, the assay should be performed within one hour.
6. Add 3 drops (approximately 100 µL) of extracted sample from the Extraction Tube to the sample well on the test cassette. Avoid trapping air bubbles in the specimen well (S), and do not drop any solution in observation window. As the test begins to work, you will see color move across the membrane.
7. Wait for the colored band(s) to appear. The result should be read at 15 minutes.
Do not interpret the result after 20 minutes. Discard used test tubes and Test Cassettes in suitable biohazardous waste container.
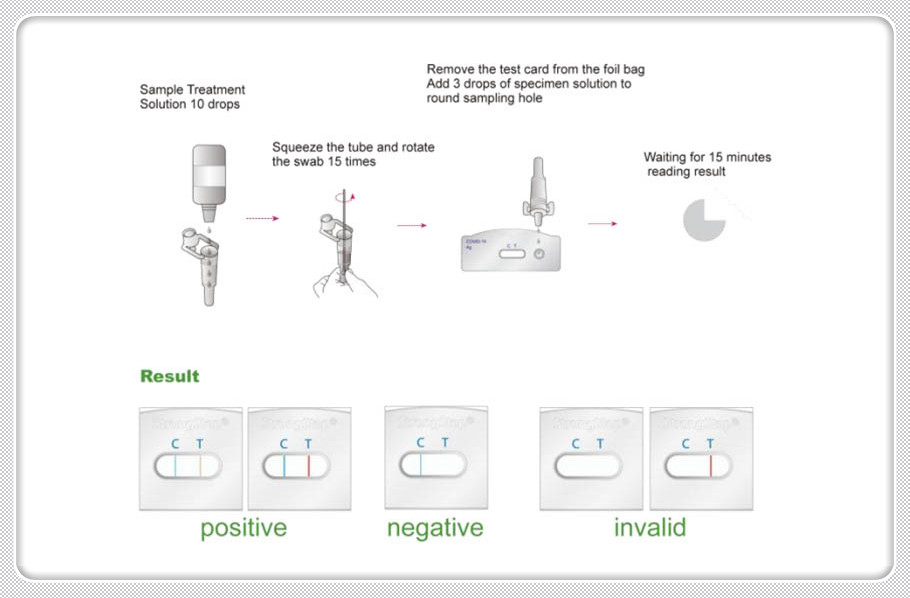
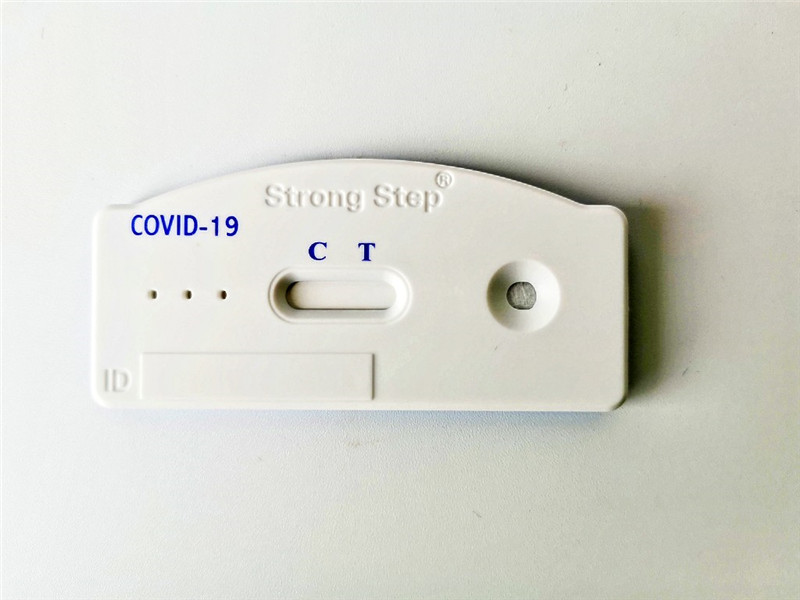
INTERPRETATION OF RESULTS
POSITIVE RESULT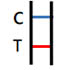 |
Two colored bands appear within 15minutes. One colored band appears in the Control Zone (C) and another colored band appears in the Test Zone (T). The test result is positive and valid. No matter how faint the colored band appears in the Test Zone (T), the test result should be considered a s positive result. |
NEGATIVE RESULT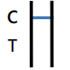 |
One colored bands appears in the Control Zone (C) within 15 minutes. No colored band appears in the Test Zone (T). The test result is negative and valid. |
INVALID RESULT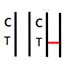 |
No colored band appears in the Control Zone (C) within 15 minutes. The test result is invalid. Repeat the test with a new test device. |
LIMITATIONS OF THE TEST
1. The test is for qualitative detection of anti-SARS-CoV-2 antigens in human Throat/Nasopharyngeal swab sample and dose not indicate the quantity of the antigens.
2. The test is for in vitro diagnostic use only.
3. As in case of all diagnostic tests, a definitive clinical diagnosis should not be based on the result of a single test but should rather be made after all the clinical findings have been evaluated, especially conjunct with SARS-CoV-2 PCR test. 4. The sensitivity for RT-PCR assay in diagnosis of COVID-19 is only 30%-80% due to poor sample quality or disease time point at the recoverd phase , etc. SARS-CoV-2 Antigen Rapid Test Device’s sensitivity is theoretically lower because of its Methodology.
GLOSSARY OF SYMBOLS
Nanjing Liming Bio-Products Co., Ltd.
No. 12 Huayuan Road, Nanjing, Jiangsu, 210042 P.R. China.
Tel: +86(25) 85288506
Fax: (0086)25 85476387
E-mail: sales@limingbio.com
Website: www.limingbio.com
Technical support: poct_tech@limingbio.com
Product packaging
Antigen Rapid Test leaflets(20200713)
COVID-19 Antigen kit
Product detail pictures:




Related Product Guide:
The organization keeps for the procedure concept "scientific administration, superior quality and effectiveness primacy, shopper supreme for 2020 Good Quality Sars-Cov-2 Pcr - SARS-CoV-2 Antigen Rapid Test – Liming Bio , The product will supply to all over the world, such as: Philippines, Mumbai, Georgia, Due to our strict pursues in quality, and after-sale service, our product gets more and more popular around the world. Many clients came to visit our factory and place orders. And there are also many foreign friends who came for sight seeing, or entrust us to buy other stuff for them. You are most welcome to come to China, to our city and to our factory!
This supplier's raw material quality is stable and reliable, has always been in accordance with the requirements of our company to provide the goods that quality meet our requirements.