2020 China New Design Sars-Cov-2 Antigen - SARS-CoV-2 Antigen Rapid Test(nasal) – Liming Bio
2020 China New Design Sars-Cov-2 Antigen - SARS-CoV-2 Antigen Rapid Test(nasal) – Liming Bio Detail:
The product has an exclusive agent in New Zealand. If you are interested in purchasing, the contact information is as follows:
Mick Dienhoff
General Manager
Phone number: 0755564763
Mobile number: 0492 009 534
E-mail: enquiries@nzrapidtests.co.nz
INTENDED USE
StrongStep® SARS-CoV-2 Antigen Rapid Test Cassette employs immunochromatography technology to detect the SARS- CoV-2 nucleocapsid antigen in human anterior nasal swab specimen. This testis single use only and intended for self-testing. It is recommanded to use this test within 5 days of symptom onset. It is supported by the clinical performance assessment.
INTRODUCTION
The novel coronaviruses belong totiie p genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel cxjronavinis are the main source of infection; asymptomatic infected people can also be an infectious source. Based on 1he current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
PRINCIPLE
The StrongStep® SARS-CoV-2 Antigen Test employs immunochromatographic test. Latex conjugated antibodies (Latex-Ab) corresponding to SARS-CoV-2 are dry-immobilized at the end of nitrocellulose membrane strip. SARS-CoV-2 antibodies are bond at the Test Zone (T) and Biotin-BSA is bond at the Control Zone (C). When the sample is added, it migrates by capillary diffusion rehydrating the latex conjugate. If present in sample, SARS-CoV-2 antigens will bind with the conjugated antibodies forming particles. These particles will continue to migrate along the strip until the Test Zone (T) where they are captured by SARS-CoV-2 antibodies generating a visible red line. If there are no SARS-CoV-2 antigens in sample, no red line is formed in the Test Zone (T). The streptavidin conjugate will continue to migrate alone until it is captured in the Control Zone(C) by the Biotin-BSA aggregating in a blue line, which indicates the validity of the test.
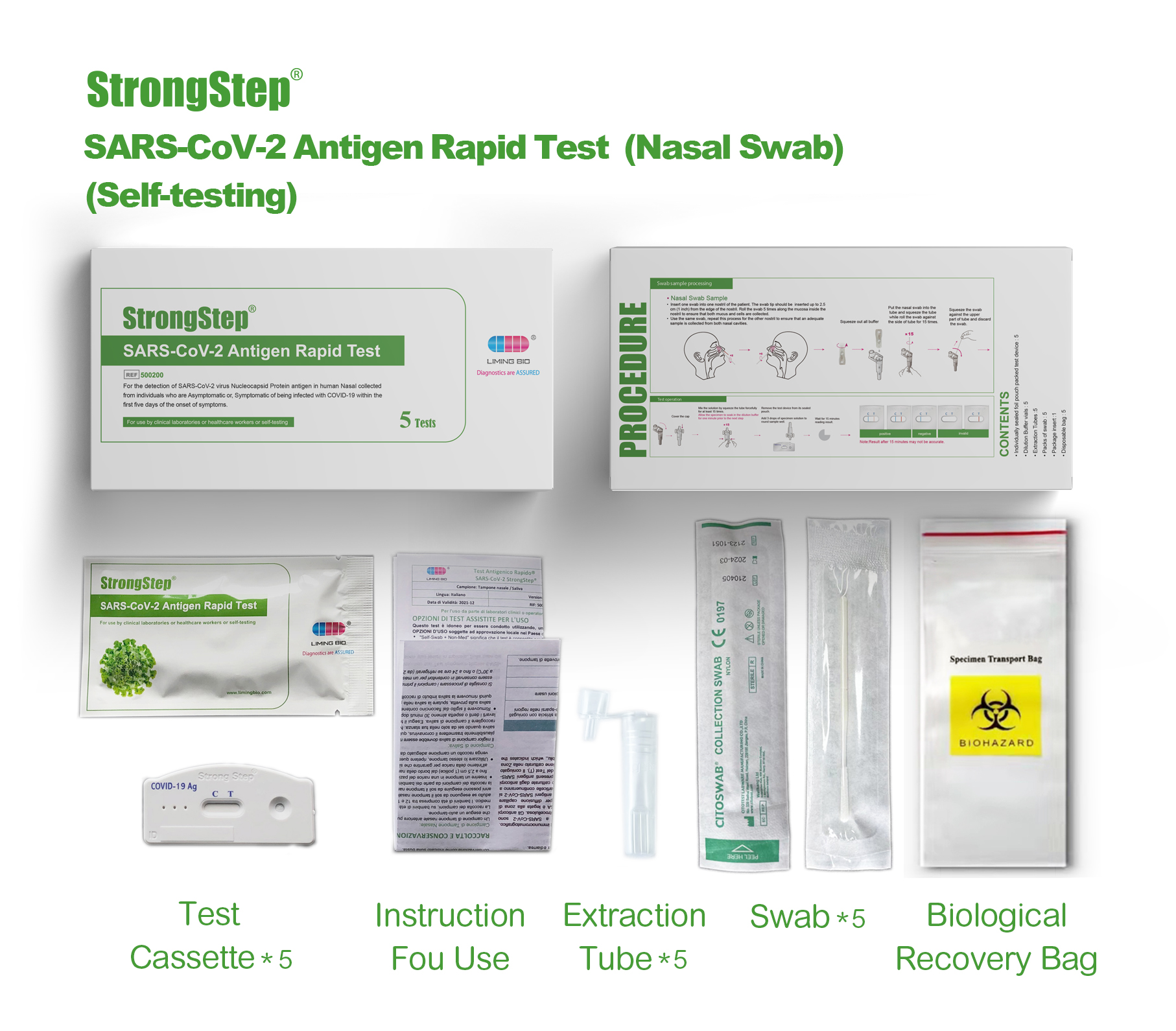
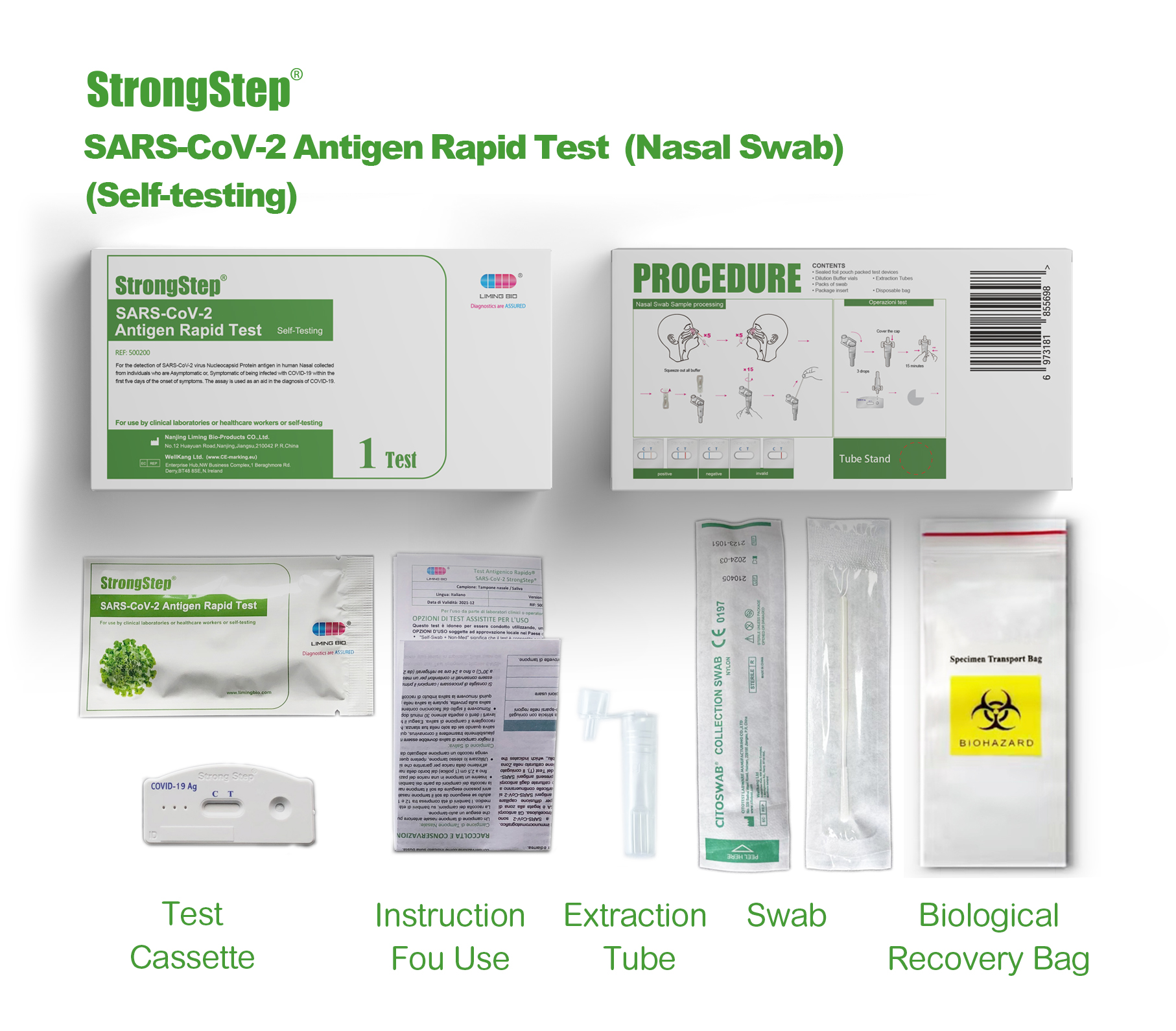
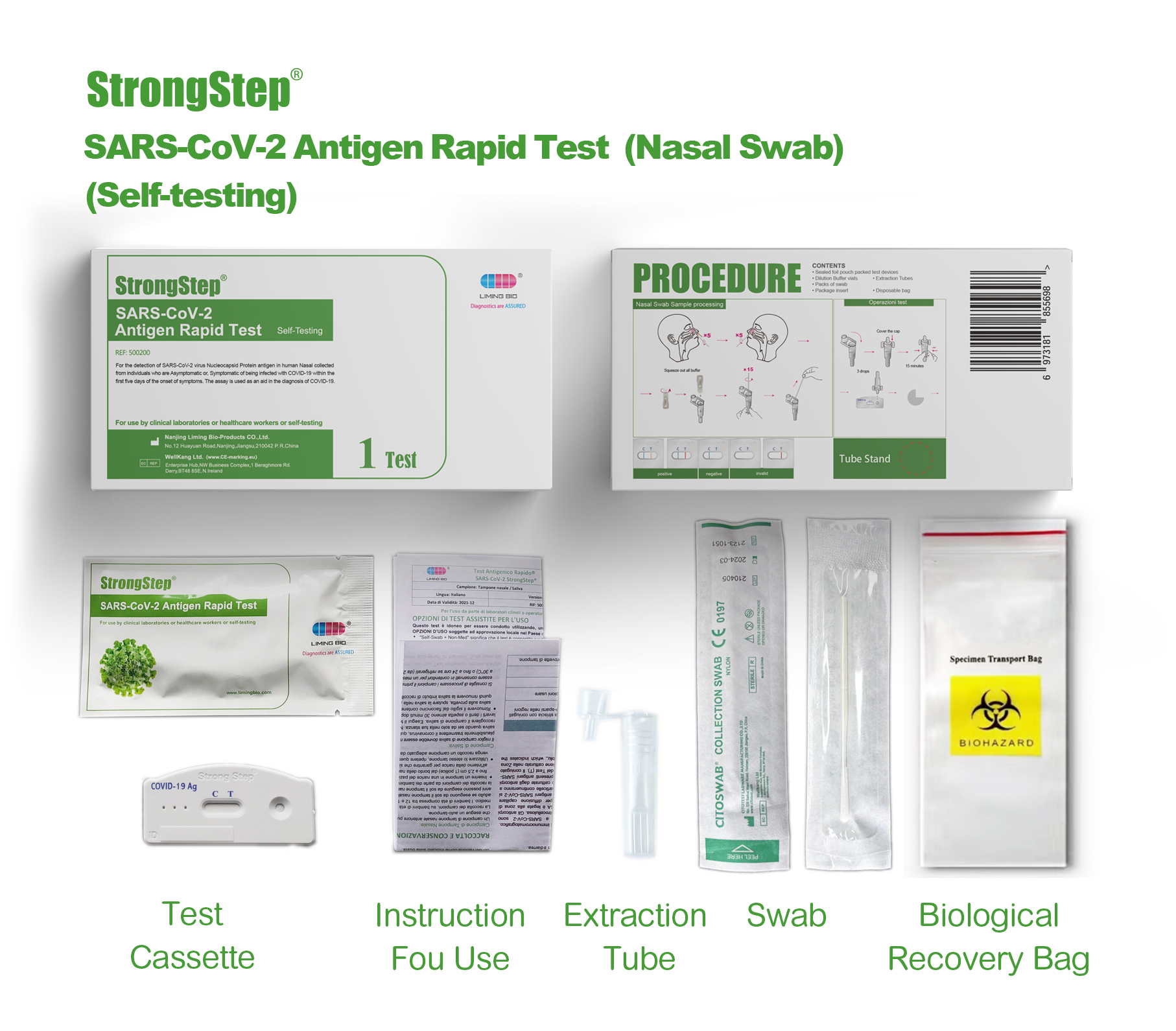
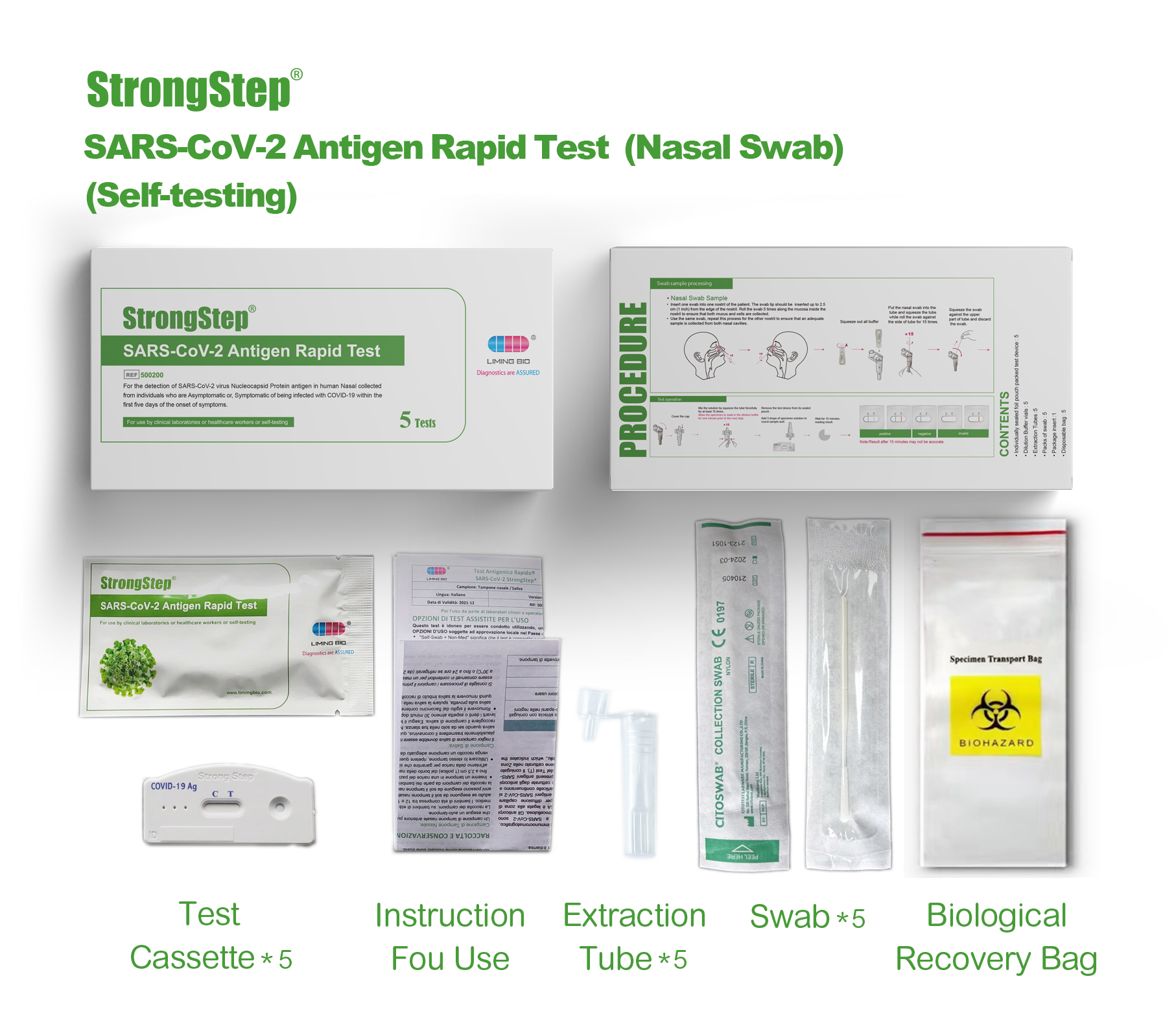
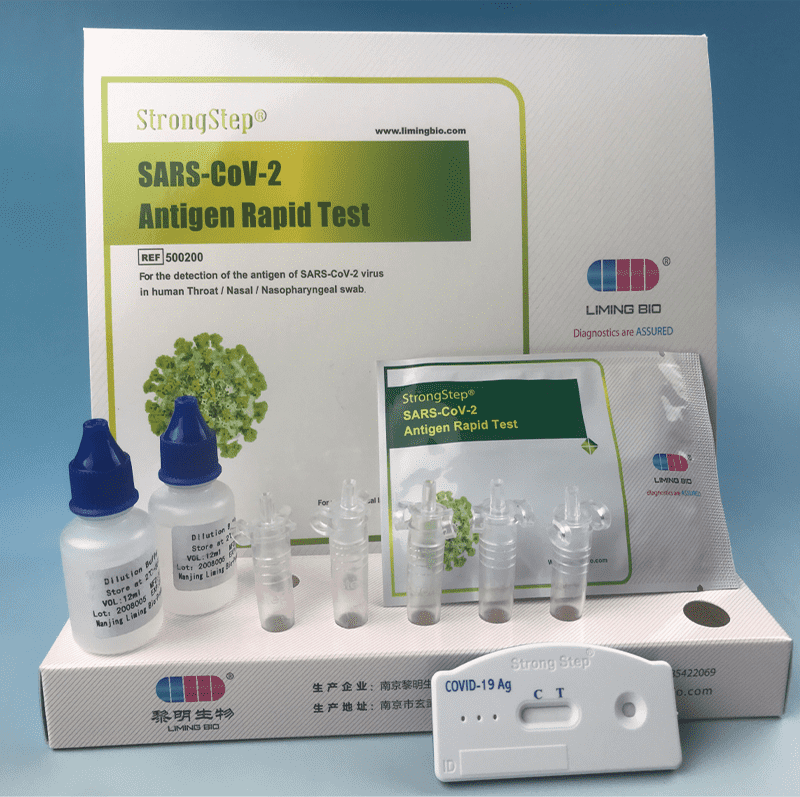
KIT COMPONENTS
1 test/box;5 tests/box:
| Sealed foil pouch packed test devices | Each device contains a strip with colored conjugates and reactive reagents pre-spreaded at the corresponding regions. |
| Dilution Buffer vials | 0.1 M Phosphate buffered saline (PBS) and 0.02% sodium azide. |
| Extraction Tubes | For specimens preparation use. |
| Packs of swab | For specimen collection. |
| Workstation | Place for holding buffer vials and tubes. |
| Package insert | For operation instruction. |
20 tests/box
|
20 Individually packed test devices |
Each device contains a strip with colored conjugates and reactive reagents pre-spreaded at the corresponding reqions. |
|
2 Extraction Buffer vials |
0.1 M Phosphate buffered saline (P8S) and0.02% sodium azide. |
|
20 Extraction tubes |
For specimens preparation use. |
|
1 Workstation |
Place for holding buffer vials and tubes. |
|
1 Package insert |
For operation instruction. |
MATERIALS REQUIRED BUT NOT PROVIDED
| Timer | For timing use. |
| Any necessary personal protective equipment |
PRECAUTIONS
-This kit is for IN VITRO diagnostic use only.
- Read the instructions carefully before performing the test.
- This product does not contain any human source materials.
-Do not use kit contents after the expiration date.
Wear gloves during the whole procedure.
STORAGE AND STABILITY
The sealed pouches in the test kit may be stored between 2-30 C for the duration of the shelf life as indicated on the pouch.
SPECIMEN COLLECTION AND STORAGE
An anterior nasal swab sample can be collected or by an Individual perfofmlng a self-swab.
Children under 18 years of age, should be performed by their aduK supervision. Adults aged 18 and over can perform the anterior nasal swab by themselves. Please follow your local guidelines for specimen collection by children.
, Insert one swab into one nostril of the patient. The swab tip should be inserted up to 2.5 cm (1 inch) from the edge of the nostril. Roll the swab 5 times along the mucosa inside the nostril to ensure that both mucus and cells are collected.
• Use the same swab, repeat this process for tha other nostril to ensure that an adequate sample is collected from both nasal cavities.
It Is recommended that specimens be processed as soon as possible after collection. Specimens can be held in container uptol hour at mom temperature (15°C to 30″C), or up to 24 hours when rsfrigeratod (2°C to 8eC) before processing.
PROCEDURE
Bring test devices, specimens, buffer and/or controls to room temperature (15-30°C) bafore use.
♦ Plac® the collected specimen Extraction tube in the designated area of the workstation.
♦ Squeeze all the Dilution Buffer into the ext radion tube.
♦ Put the specimen swab into the tube. Vigorously mix the solution by rotating the swab forcefully against the side of the tube for least 15 times (while submerged). Best results are obtained when the specimen is vigorously mixed in the solution.
♦ Allow the swab to soak in the Extraction Buffer for one minute prior to the next Step.
♦ Squeeze out as much liquid as possible from the swab by pinching the side of the flexible extraction tube as the swab is removed. At least 1/2ofttie sample buffer solution must remain in the tube for adequate capillary migration to occur. Put the cap onto ihe extracted tube.
♦ Discard the swab in a suitable biohazardous waste container.
♦ The specimens extracted can retain at room temperature for 30 minutes without affecting the result of the test.
♦ Remove ths test device from its sealed pouch, and place it on a dean, level surface. Label the device with patient or control identification. To obtain a best result, the assay should be performed within 30 minutes.
♦ Add 3 drops (approximately 100 pL) of extracted sample from the Extraction Tube to the round sample well on the test device.
Avoid trapping air bubbles In the sample well (S), and do not drop any solution In observation window. As the test begins to work, you will see color move across the membrane.
♦ Wart for the colored band(s) to appear. The result should be read by visual at 15 minutas. Do not interpret the result after 30 minutes.
• Put the test tube containing the swab and the used test device Into theattached biohazard bag and seal it, and then discard it in a suitablebiohazard waste container. Then throw away the remaining Items
• Wash your hands or reapply hand sanitizer.
Discard used Extraction Tubes and Test Devices in suitable biohazardous waste container.
V2.0_00.png)
INTERPRETATION OF RESULTS
LIMITATIONSOF THE TEST
1- The kit is intended to use for the qualitative detection ofSARS-CoV-2 antigens from Nasal.
2.This test detects both viable (live) and non-viable SARS-CoV-2. Test performance depends on the amount of virus (antigen) in the sample and may or may not correlate with viral culture results perfonned on the same sample.
3.A negative teat result may occur if the level of antigen in a sample is below the detection limit of the test or if the sample was collected or transported improperly.
4.Failure to follow the Test Procedure may adversely affect test performance and/or invalidate the test result.
5.Test results must be correlated with the clinical history, epidemiological data, and other data available to the clinician evaluating the patient.
6.Positive test results do not rule out co-infections with other pathogens.
7.Negative test results are not intended to rule in other non-SARS viral or bacterial infections.
8.Negative results from patients with symptom onset beyond seven days, should be treated as presumptive and confirmed with an local FDA authorized molecular assay, if necessary, for clinical management, including infection control.
9.Specimen stability recommendations are based upon stability data from influenza testing and performance may b© different with SARS-CoV-2. Users should test specimens as quickly as possible after specimen collection.
10.The sensitivity for RT-PCR assay in diagnosis of COVID-19 is only 50%-80% due to poor sample quality or disease time point at the recoverd phase,etc.SARS-CoV-2 Antigen Rapid Test Device’s sensitivity is theoretically lower because of its methodology.
11.In order to get enough virus, it is suggested to use two or more swabs to collect different sites of sample and extract all the sampled swab in the same tube.
12.Positive and negative predictive values are highly dependent on prevalence rates.
13.Positive test results are more likely to represent false positive results during periods of little I no SARS- CoV-2 activity when disease prevalence is low.False negative test results are more likely when prevalence of disease caused by SARS-CoV-2 is high.
14.Monoclonal antibodies may fail to detect, or detect with less sensitivity,SARS-CoV-2 influenza viruses that have undergone minor amino acid changes in the target epitope region.
15.The performance of this test has not been evaluated for use in patients without signs and symptoms of respiratory infection and parformance may differ in asymptomatic individuals.
16.The amount of antigen In a sample may decrease as the duration of Illness Increases. Specimens collected after day 5 of illness are more likely to be negative compared to a RT-PCR assay.
17.Sensitivity of the test after the first five days of the onset of symptoms has been demonstrated to decrease as compared to a RT-PCR assay.
18.It is suggested to use StrongStep® SARS-CoV-2 IgM/IgG antibody rapid test (caW 502090) to detect the antibody to increase the sensitivity of diagnosis of COVID-19.
19.It Is not recommend to use Virus Transportation medla(VTM) specimen In this test, If customers Insist to use this sample type, customers should validate themselves.
20.The StrongStep® SARS-CoV-2 Antigen Rapid Test was validated with the swabs provided in the kit. Use of alternative swabs may result In false results.
21.Frequent testing is necessary to increase the sensitivity of diagnosis of COVID-19.
22.No drop off in sensitivity when compared with the wild type with rasped to the following variants – VOC1 Kent, UK, B.1.1.7 and VOC2 South Africa, B.1.351.
23 Keep out of reach of children.
24. Positive results indicate that viral antigens were detected in the sample taken, please Self-quarantine and Inform your family doctor promptly.
1V2.0_01_副本.jpg)
Nanjing Liming Bio-Products Co., Ltd.
No. 12 Huayuan Road, Nanjing, Jiangsu, 210042 P.R. China.
Tel: +86(25) 85288506
Fax: (0086)25 85476387
E-mail: sales@limingbio.com
Website: www.limingbio.com
Technical support: poct_tech@limingbio.com
Product packaging
Antigen Rapid Test leaflets(20200713)
SARS-CoV-2 Antigen Rapid Test German Manual
saliva sars-cov-2 Antigen clinical study(Swab)
SARS-CoV-2-Antigen-Rapid-Test(Anterior-nasal-swab)
COVID-19 Antigen kit
Product detail pictures:



Related Product Guide:
We depend on sturdy technical force and continually create sophisticated technologies to meet the demand of 2020 China New Design Sars-Cov-2 Antigen - SARS-CoV-2 Antigen Rapid Test(nasal) – Liming Bio , The product will supply to all over the world, such as: Mexico, Cancun, Johannesburg, We adhere to the honest, efficient, practical win-win running mission and people-oriented business philosophy. Excellent quality, reasonable price and customer satisfaction are always pursued! If you are interested in our products, just try to contact us for more details!
Perfect services, quality products and competitive prices, we have work many times, every time is delighted, wish continue to maintain!

V2.01_00_副本.jpg)